Hematogenous small intestinal metastasis 8 months after removal of epithelial ovarian cancer: a case report
Introduction
Ovarian cancers usually spread in four different ways: Trans peritoneal spread, direct invasion, lymphatic, and hematogenous. The primary way of metastasis of ovarian carcinoma is considered to be direct peritoneal dissemination in the abdominal cavity and serosa of bowel. Conversely, hematogenous dissemination is rare, and seldom leads to gastrointestinal metastasis. We report a case of a patient who was operated for an epithelial ovarian cancer and presented with small intestinal metastasis 8 months later. Vascular cancer emboli could be detected by microscopy. Immunohistochemistry tests proved the small intestinal metastasis originated from primary ovarian cancer.
Case presentation
In January 2017, a 64-year-old female, unmarried and childless, visited our department for abdominal distension. Ultrasonic examination showed there was a mass in the left suprapubic area with large quantities of ascites. The blood tumor marker levels were mildly elevated in the following measures: carbohydrate antigen (CA) 19-9: 140.5 U/mL (reference range, 0 to 22 U/mL), CA125: 80.86 U/mL (reference range, 0 to 35 U/mL), carcinoembryonic antigen (CEA): 5.57 ng/mL (reference range, 0 to 3.4 ng/mL), CA15-3: 38.13 U/mL (reference range, 0 to 21 U/mL), alpha fetoprotein (AFP): 5.15 ng/mL (reference range, 0 to 8 ng/mL). Meanwhile, the CT results of epigastrium and chest were negative.
The patient underwent a total hysterectomy, a bilateral adnexectomy and a major mass removal in the pelvic cavity. Because of severe bleeding, an omentectomy, appendectomy, pelvic lymphadenectomy and para-aortic biopsy were all performed three months later. Pathological examination revealed the ovarian cancer was high-grade serous adenocarcinoma, and stage III according to the FIGO staging system, due to the invasion of the omentum and peritoneum, and the lymph node were not involved. She received two periods of adjuvant chemotherapy, using paclitaxel liposome and paraplatin between the two operations.
After the second operation, the patient’s laboratory outcomes showed no abnormality before the sixth period of chemotherapy, when she complained of abdominal distension, nausea, vomiting and inability to eat.
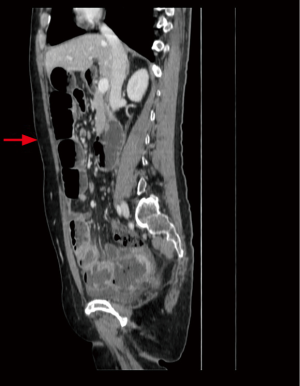
The patient underwent an enhanced computed tomography (CT) scan of the abdomen and pelvis, which demonstrated incomplete intestinal obstruction (Figure 1). However, the tumor markers CA199, CA125, and HE4 were all within normal range before each chemotherapy. She grew increasingly feeble because of her inability to eat and vomiting for a week.
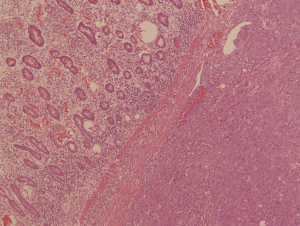
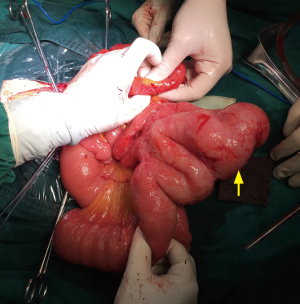
In September 2017, she visited our department and underwent an exploratory laparotomy. There were a few metastatic lesions and intestinal adhesion in the abdominopelvic cavity. However, a 6 cm tumor was detected 0.5 m away from the Treitz ligament, and another 2.5 cm tumor was found 1.5 m away from Treitz ligament, both of which were protruding into the small intestine lumen, but not invading the intestinal serosa (Figure 2). The 6 cm mass could explain the intestinal obstruction. About 80 cm of small intestine was removed and anastomosed. She recovered to consuming fluids 5 days after the third operation, and then turned to soft food.
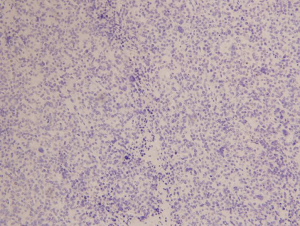
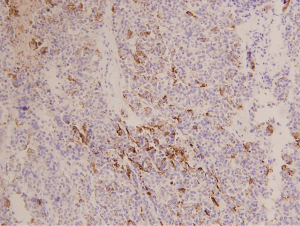
The pathologic examination showed that the two masses were high-grade serous adenocarcinoma (Figure 3). The immunohistochemical stainings were negative for CK 20 (Figure 4) and positive for CK 7 (Figure 5). Considering the history of ovarian carcinoma, this examination supported the idea that the intestinal tumor was derived from the ovary. Moreover, 8 mesenteric lymph nodes in the excised mesentery were uninvolved, but tumor thrombus could be seen in the vessels. We were inclined to hypothesize that the intestinal metastasis was the result of the hematogenous dissemination.
Follow-up
The patient had to stop chemotherapy because of an adverse drug reaction; meanwhile, CT showed several hepatic metastases two months after the third operation. Her body condition was too poor to be treated with hospice care.
Discussion
In 2017, 14,080 deaths out of a total number of 22,440 cases of ovarian cancer were reported in United States (1). Ovarian carcinoma is relatively rare, but it is the most lethal gynecological malignant tumor. Of the different histological types, epithelial ovarian cancer accounts for 90% of ovarian cancer. The most frequent witnessed are the high-grade serous type carcinomas, which accounts for 67% of all ovarian cancer. Eighty-eight percent of ovarian carcinoma are stage III or IV when diagnosed (2). Even though about 90% of patients in the early stages are able to survive more than 5 years (3), unfortunately, the symptoms of early ovarian cancer tend to be non-specific, and easy to escape notice. Much progress has been made to surgery and treatment of ovarian mass in recent years, but the 5-year survival rate is less than 30%, which mostly results from diagnosis being made only until the late stages (4).
Although small intestine tumors are rare, only accounting for 2% of all primary gastrointestinal tumors (5), metastasis of the small intestine, which is infrequent, accounts for 10% of all intestinal malignancies. The intramucosal localization of intestinal metastasis is even rarer (6). Primary adenocarcinomas of the small intestine do exist, and they need to be distinguished from metastasis of ovarian cancer, as the therapies for both tumors are distinctly different.
It is difficult to identify the difference between intestinal and ovarian serous adenocarcinoma by sight alone, and so immunohistochemical staining is very helpful to distinguish the origin. In our case, the CK staining and recent history of ovarian cancer oriented the diagnosis towards an ovarian origin. The Immunohistochemical staining for CK 7 and CK 20 is useful for the identification for intestinal and ovarian cancer. Our staining showed CK 7 positive and CK 20 negative, which is also usually the case with serous ovarian carcinomas, as opposed to gastrointestinal cancer which typically demonstrates the opposite result (7-9).
The dissemination of ovarian carcinoma to the gastrointestinal tract was reported in four different ways.
Firstly, it primarily disseminates within the peritoneal cavity and is only superficially invaded. Ovarian cancer cells are carried by the physiological movement of the peritoneal fluid to all organs in the peritoneal cavity, including the diaphragm, serosa of intestines, omentum, and the entire peritoneum (3,7).
Secondly, the tumor can gradually invade adjacent structures. The most frequent locations are in the colon, uterus and fallopian tubes (8).
The third way of dissemination can be achieved by the lymphatic route. Para-aortic lymph nodes are the first group to be involved, followed by broad ligaments lymph nodes, which drain into the hypogastric lymph nodes. The third group consists of the external iliac and inguinal chains, which are rarely involved (9,10).
Finally, hematogenous dissemination leads to invasion of the pleura, liver, lung. Spread to the kidneys is generally rare (10,11).
Dauplat et al. (12) and Cormio et al. (11) revealed that the risk of distant metastases in epithelial ovarian cancer is related to the presence of ascites, the staging of disease, the histologic features of the tumor, the extent of initial surgery, and the lymph node involvement. They reported that the metastases may occur anywhere, and the most commonly involved sites are the pleura, liver, lungs, central nervous system, spleen, skin, bone, and breast. Dauplat et al. (12) reported that the interval time between diagnosis of ovarian cancer and documentation of metastases was 1 to 139 months and survival time after diagnosis of distant metastases was 1 to 51 months. Similarly, Cormio et al. (11) reported interval and survival times were 3 to 105 months and 1 to 58 months respectively.
Manzoni (6) reported a case similar to ours. He described an intestinal metastasis of an ovarian malignant mixed Müllerian tumor that was limited to the intestinal mucosa. The metastasized mass was large enough to occlude in the intestinal lumen, and the primary origin was found to be associated with metastasis, but it is unclear which route the metastasis took.
In our case, the integrity of the intestinal serosa and the 8 mesenteric lymph nodes in the excised mesentery were all negative, but tumor thrombus could be seen in the vessels. We were inclined to hypothesize that the intestinal metastasis come from the hematogenous metastasis, which is rare in ovarian carcinoma according to the literature. The interval time between diagnosis and metastasis was 8 months.
However, another study showed that hematogenous metastasis of ovarian cancer was always delayed. Trastour (9) reported a case of hematogenous rectal metastasis of a clear cell carcinoma of the ovary 20 years after removal of epithelial ovarian cancer. In addition to this, Bijek (13) reported a case of hematogenous rectal metastasis after 9 years. Researchers found circulating tumor cells in the blood samples of ovarian cancer patients, suggesting a blood-borne route of ovarian carcinoma metastasis (4,14), although small intestine is a rare site among the metastases (5). In our case, the hematogenous intestinal metastasis occurred earlier than the hepatic metastasis, which developed 8 months after the first operation of the primary tumor. This controversy can be solved through accumulating more clinical data.
Other tumor markers, such as CA125, human epididymis protein (HE4) can be used. A value of CA125 between 10 and 35 U/mL indicates a relative risk of recurrence for postoperative patients of epithelial ovarian carcinoma (15), while the rising HE4 value can predict recurrent epithelial ovarian cancer with specificity of 95.6%, and a sensitivity of 52.7%. However, in our case, the values of CA125 and HE4 were always normal. As such, it was difficult to judge whether the cancer recurrent or not, and it was necessary that serological testing combine with imaging examination. In this case, there are several questions that should be further considered.
iMDT
Hematogenous dissemination of ovarian carcinoma is rare and late. The interval between diagnosis of ovarian cancer and detection of hematogenous small intestinal metastasis is only 8 months. Is it related to the first two operations?
Expert opinion 1
Although currently adopted FIGO staging system do not take in to account way of dissemination, hematogenous dissemination is associated with stage IV disease (i.e., hepatic and splenic nodules). Reading this case seems that the patients was submitted to interval debulking surgery after a suboptimal debulking. Hematogenous dissemination might be the cause of the small bowel nodules and hepatic dissemination. No mention of preoperative workup is reported. I’m concerned that these lesions would be present at the time of primary and interval debulking surgery.
Expert opinion 2
This case report illustrates the aggressive nature of high grade serous epithelial ovarian cancer. Pre-operative staging investigations are not well outlined in the manuscript. It would be interesting to know if there were pleural effusions or intra-hepatic disease at the time of diagnosis of the bowel obstruction.
Haematogenous spread is less common than the usual transcoelomic intra-abdominal spread but the true incidence is generally underestimated (14). In pathological specimens, lymph vascular space involvement is often reported and that may be interpreted as an early manifestation of vascular metastases.
Optimal cytoreduction (removing all visible disease) remains one of the most important predictors of survival. Thorough surgery as early as possible is essential to improve outcome. Surgery may increase likelihood of small bowel obstruction due to adhesions but there is no clear evidence that surgery increase haematogenous spread of epithelial ovarian cancer.
Expert opinion 3
I do not think so. If this was truly hematogenous, it was inevitable.
The patient received 6 cycles of chemotherapy in all, but the effect was not satisfactory. Based on prognosis and quality of life, how should clinician decide on an individualized treatment plan?
Expert opinion 1
The 4th Consensus Conference on Ovarian Cancer held in Vancouver, Canada in 2010 established four categories of recurrence for ovarian cancer. Patients are classified in platinum-refractory, resistant, partial sensible and sensible according to the time lasing between last dose of platinum and recurrence. This classification had important implication from both prognostic and therapeutic point of view. Prognosis of patients recurring in the first weeks after chemotherapy is poor, regardless type of chemotherapy administered. Further innovative trials are needed to identify new effective treatment modality.
Expert opinion 2
The standard therapies for epithelial ovarian cancer include debulking surgery and chemotherapy. The most widely used combination is carboplatin and a taxane. This patient had a biochemical and clinical response to chemotherapy because her tumor markers normalized and the ascites cleared. The response was partial and not long lasting. In this situation (platinum resistance) there is often an intrinsic drug resistance in the tumor cells (16). Changing to a second line combination of chemotherapy or adding an angiogenesis inhibitor may improve response. However, Platinum resistance is a poor prognostic indicator and is likely due to the inherent aggressive nature of the tumor.
Expert opinion 3
Unfortunately, she had platinum resistant ovarian cancer and her prognose was poor. Indeed, many clinical trials indicate that overall survival is less than 12 months. Therefore, quality of life should be emphasized in general.
Targeted therapy is the focus of treatment of tumors, are genetic tests and targeted therapies for ovarian cancer far away?
Expert opinion 1
Recently, growing data suggested the immunotherapy and target therapy is effective in patients with newly diagnosed and recurrent ovarian cancer. Precision medicine based on genomic-based therapy would be the new standard of care for our patients. But we have to wait results from ongoing phase I and II trials. Further evidence is warranted.
Expert opinion 2
What clinicians call epithelial ovarian cancer is in fact a widely varying group of individual tumors. We know that many of these tumors originate from the Fallopian tubes or the peritoneum. Genetic variation may be the reason why tumors respond to platinum-based chemotherapy or not and also may influence whether optimal surgery is possible. Genetic studies can help to target the cancer therapy better. One example is the use of poly (ADP-ribose) polymerase (PARP) inhibitors in the management of patients with epithelial ovarian cancer who have known mutations in BRCA genes. Many oncogenic pathways have been identified for possible targeted treatment. Powerful, immune-based cancer treatments are now in development and tested in clinical trials and will likely become part of routine management in the next few decades.
Expert opinion 3
Orapalib is extremely effective in patients with BRCA mutations. Pembrolizumab is effective in patients with MSI-High. Therefore, I think genetic test should be introduced into clinic as soon as possible.
Conclusions
For intractable patient, iMDT can provide advanced treatment perspectives and therapies. On the other hand, for clinicians, iMDT discussion provide a platform for information sharing and communicating.
In this case, hematogenous small intestinal metastasis is rare, clinicians should consider the possibility of hematogenous intestinal metastasis when a patient with an ovarian carcinoma history shows signs of intestinal obstruction. This case could help surgeons have a better understanding about the metastasis of epithelial ovarian cancer, especially via hematogenous manner, in addition to the site of the metastasis, as well as the variations of the tumor markers during follow-up.
For the risk of high recurrence rate, regular follow-up is very important. Even if tumor markers are normal, when clinical manifestations suggest gastrointestinal involvement, abdominal examinations, including exploratory laparotomy, should be carried out.
Acknowledgments
Pathological data provided by Yangmei Shen, imaging data provided by Zhimin Zhao.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “iMDT Corner”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm.2019.01.01/coif). The series “iMDT Corner” was commissioned by the editorial office without any funding or sponsorship. GB serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Sep 2018 to Aug 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nam G, Lim YM, Cho MS, et al. Skin metastases in ovarian clear cell adenocarcinoma: a case report and a review of the literature. Obstet Gynecol Sci 2017;60:593-7. [Crossref] [PubMed]
- Köbel M, Huntsman D. Molecular Pathology of Ovarian Carcinomas. Surg Pathol Clin 2011;4:275-96. [Crossref] [PubMed]
- Seidman JD, Kurman RJ. Pathology of ovarian carcinoma. Hematol Oncol Clin North Am 2003;17:909-25. vii. [Crossref] [PubMed]
- Yeung TL, Leung CS, Yip KP, et al. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol 2015;309:C444-56. [Crossref] [PubMed]
- Paski SC, Semrad CE. Small bowel tumors. Gastrointest Endosc Clin N Am 2009;19:461-79. [Crossref] [PubMed]
- Manzoni M, Pagni F, Perego P, et al. Large polyp of the small intestine: an unexpected metastasis of ovarian carcinosarcoma. Int J Surg Pathol 2013;21:506-7. [Crossref] [PubMed]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053-64. [Crossref] [PubMed]
- Kono M, Nagami Y, Ominami M, et al. A Metastatic Gastric Tumor from Ovarian Cancer. Intern Med 2018;57:345-9. [Crossref] [PubMed]
- Trastour C, Rahili A, Schumacker C, et al. Hematogenous rectal metastasis 20 years after removal of epithelial ovarian cancer. Gynecol Oncol 2004;94:584-8. [Crossref] [PubMed]
- Friedman M, Browde S, Rabin S, et al. Late metastases of ovarian carcinoma. A case report. S Afr Med J 1984;65:178-9. [PubMed]
- Cormio G, Rossi C, Cazzolla A, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer 2003;13:125-9. [Crossref] [PubMed]
- Dauplat J, Hacker NF, Nieberg RK, et al. Distant metastases in epithelial ovarian carcinoma. Cancer 1987;60:1561-6. [Crossref] [PubMed]
- Bijek JH, Ehnart N, Mathevet P. Hematogenous dissemination in epithelial ovarian cancer: case report. J Gynecol Obstet Biol Reprod (Paris) 2011;40:465-8. [Crossref] [PubMed]
- Pradeep S, Kim SW, Wu SY, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell 2014;26:77-91. [Crossref] [PubMed]
- Guo N, Peng Z. Does serum CA125 have clinical value for follow-up monitoring of postoperative patients with epithelial ovarian cancer? Results of a 12-year study. J Ovarian Res 2017;10:14. [Crossref] [PubMed]
- Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol 2011;12:1169-74. [Crossref] [PubMed]
Cite this article as: Deng C, Liu D, Bogani G, Botha MH, Guo T. Hematogenous small intestinal metastasis 8 months after removal of epithelial ovarian cancer: a case report. Gynecol Pelvic Med 2019;2:1.