
Intramural fibroid and fertility—to operate or not
Introduction
Uterine fibroid also called leiomyoma is the commonest monoclonal tumour in women. 70–80% of women will develop fibroid by the age of 50 (1). Thirty percent of infertile patients have fibroids. Fibroids may be the sole cause of infertility in 2–3% of women (2). Current literature calls for removal of submucous fibroid and possibly cavity distorting intramural fibroids to optimize pregnancy outcome (3). However, removal of non-cavity distorting (NCD) intramural fibroids is still controversial. This review is aimed at looking at the current literature on this type of fibroids. We aim to answer the following questions:
- What are the classifications of fibroids?
- What is the pathophysiology that causes subfertility in patients with intramural fibroids?
- Does intramural fibroid cause infertility?
- Does myomectomy improve pregnancy rates?
- Are there alternatives to myomectomy in these patients?
Classification of fibroids
Traditionally fibroids are classified by its location in the uterus. They are classified as cervical, submucous (SM), subserosal (SS) and intramural (IM) fibroids (4,5).
Based on the International Federation of Gynaecology and Obstetrics (FIGO) systems, fibroids are classified into several types (6). Based on this FIGO classification, Type 0 and 1 are SM fibroids. Type 2 although >50% is IM has been classified as SM as well. Type 3 is an IM fibroid and it is distinguished from type 2 by doing a hysteroscope with the lowest possible intrauterine pressure necessary to allow visualization. There should not be any bulge into the endometrial cavity. Type 4 is the classical IM fibroid. Type 5 even though >50% is IM is often classified as SS. Types 6 and 7 are SS fibroids.
Based on this FIGO classification, an IM fibroid should be only type 3 and 4. The average thickness of the myometrium at the body of the uterus ranges from 1.5 to 3 cm (7,8). Even though a fibroid can expand the thickness of the myometrium and remain type 3 and 4, there is a high chance that a fibroid >3 cm may have SM or SS component. We believe that most published literature on IM fibroids may have included type 5 fibroids and some even would have included type 2 fibroids because the SM component was not clearly seen on ultrasound and/or hysteroscopy.
Pathophysiology
Studies show that uterine fibroid causes endometrial vascular disorders and inflammation resulting a non-conducive environment for embryo implantation and thus leading to infertility (9,10). The way IM fibroid causes difficulty in conceiving has been discussed extensively in the paper by Pier and Bates (11). The following discussion on the pathophysiology of IM fibroid causing subfertility is extracted from this paper.
Implantation
Implantation is a complex process. Several factors such as HOXA-10, glycodelin, leukemia inhibitory factor and glutathione peroxidase 3 are involved in this process.
HOXA-10 is responsible for cellular differentiation while glycodelin is responsible in promoting angiogenesis, suppressing natural killer cells (NKc) and inhibiting the binding of the spermatozoa to the zone pellucida. Normally, both factors reduce during follicular phase and increase during implantation. With the presence of IM fibroids, both HOXA-10 and glycodelin were reduced during implantation (12-19). Although studies which showed a reduction trends in HOXA-10 is not conclusive but this reduction causing inability of the embryo to implant is confirmed in animal model (12-15). So, we hypothesis that the reduction of both factors is responsible in embryo implantation failure causing infertility.
JZ
In women of reproductive age, magnetic resonance imagining (MRI) has shown three distinct layers in the myometrium. The innermost layer that immediately abuts the endometrium is labelled the JZ (20). This zone may affect fertility by two different mechanisms. Firstly, the origin of myometrial peristalsis in the JZ (21). Disruption of this zone by fibroids may lead to increased peristalsis (22). This will be discussed in the next section. Secondly, IM fibroids may cause thickening or disruption of the JZ leading to poor reproductive outcome (22).
The changes in the thickness of the JZ can be due to the changes in the expression of oestrogen, progesterone, the respective receptors and aromatase (23-25). Besides, we noticed that the amount of NKc and macrophage cells in the uterine influence the fertility potential. Study demonstrated that the NKc were significantly reduced while the macrophage cells were significantly increased in the endometrium closer to the fibroid compared to other area, the amount of both cells were significantly reduced regardless of the area when compared to the fibroid-free patient (26).
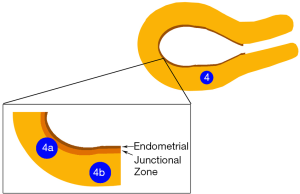
Since JZ plays an important role in implantation and its disruption may lead to implantation failure, we propose that type 4 fibroid can be further classified into type 4a and 4b as shown in Figure 1. 4a are fibroids that disrupt the JZ but does not reach the endometrium and type 4b are fibroids that do not disrupt the JZ.
Uterine myometrial peristalsis
There are 2 types of uterine contractions. The first is focal and sporadic bulging of the myometrium first described by Togashi et al. (24). The second is the rhythmic and subtle stripping movement in the subendometrial myometrium known as uterine peristalsis (UP) captured by cine mode magnetic resonance imaging (cMRI) (25).
From menstruation to the mid-ovulatory phase of the menstrual cycle, the uterus contracts from the cervix to the fundus with increasing frequency. Post-ovulation, the contraction frequency decreases to relatively quiet during implantation. In the luteal phase, the direction of peristalsis is reversed (26). Based on the studies, UP is increased in patients with IM and SM fibroids during the mid-luteal phase and decreased during the peri-ovulatory phase compared to the healthy controls (27,28).
The relationship between infertility and abnormal UP among patients with IM fibroids was explored by Yoshino et al. (29). Ninety-five infertile patients with only IM fibroids underwent cMRI during the implantation period (luteal phase days 5–9) and were further categorized into two groups (low and high uterine peristaltic frequency). Low uterine peristaltic frequency was categorized as having <2 peristalsis in 3 mins while high frequency was categorized as having ≥2 movements within 3 mins. To avoid bias, the authors recruited patients with same numbers and diameter of fibroid but half of them had cavity-encroaching fibroids. They offered infertility treatment like natural cycle, ovulation induction by hormonal therapy and intrauterine insemination in an increasing manner depends on the severity of the infertility. Results showed 34% of pregnancy rate in the low-frequency group while 0% in the high-frequency group within 2 years post-treatment. This demonstrates that abnormal UP is a likely cause of infertility. However, why some IM and even SM fibroids cause high frequency peristalsis while other doesn’t is not known.
Since UP is at the JZ, disruption of this zone by fibroid may further increase UP (18). So, one can postulate that type 3 and type 4a fibroids may have more UP than type 4b fibroids leading to more subfertility. This needs to be further evaluated by clinical studies. There are several methods of measuring UP namely intrauterine pressure measurement, transvaginal ultrasound and cMRI. Each method has its own advantages and disadvantages (30). Measuring UP accurately and inexpensively will assist in determining which patients with IM fibroid will benefit from treatment.
Fibroid pseudocapsule
Leiomyoma is covered by a thin layer which can be identified easily during myomectomy, known as fibroid pseudocapsule (PC). This layer contains bundle of smooth muscle cells and neurotransmitters. Besides, it is highly vascular to supply blood to the myoma and allows neovascularization to occur (31). This statement is proven in studies showing an upregulation of endogolin and CD34 (marker of neovascularization) in the PC compared to the fibroid and surrounding myometrium (32,33).
Thickness of the PC varies with the type and location of the fibroid which may alter the expression of modulators. It is significantly thicker in SM than IM fibroids while significantly thicker in IM than SS fibroids. The thickness also increases as the fibroid approaches to the cervix resulting an increase in the expression of enkephalin and oxytocin which will alter the UP and affect fertility (34). The altered UP may also be contributed by the high levels of neurotensin, neuropeptide tyrosine, and protein gene product 9.5 present in the IM fibroid PC (35). The presence of PC and the associated cytokines, growth factors and hormones may be responsible for the abnormal UP which may result in pregnancy complications like premature uterine contraction resulting in preterm delivery in women with large IM fibroids (36).
Although these neurotransmitters produced by PCs induce UP, they are important in promoting inflammation and proper wound healing. So, it is mandatory to perform intracapsular myomectomy without excising the PC to reduce intraoperative blood loss, enhance better uterine healing and correct musculature anatomical restoring to preserve the uterine functionality for reproductive purpose (37-39).
Steroid hormones
Uterine fibroid does not present in pre-puberty and rarely post-menopausal with low incidence in multiparity and late menarche (40). This implies that fibroid development depends on the hormonal status. Ovarian steroids, oestrogen (E2) and progesterone (P4) are responsible for the formation and growth of the fibroid (41). Fibroid tissues have a higher concentration of aromatase, E2, oestrogen receptors α and β, and progesterone receptors (PR) compared to the surrounding healthy myometrium (41,42).
Fibroids are known as E2-dependent tumour since E2 is the primary growth promoter of fibroids (43,44). High level of E2 decreases the tumour suppressor protein (p53) in the fibroid cells and regulates its growth factors and signalling pathways, stimulating cellular proliferation and fibroid growth (45). Besides, E2 is important in maintaining the progesterone receptor (PR-A and PR-B) level to induce the PR ligands action and subsequently mediate the P4 actions (43,45).
Previous studies have shown that the growth of fibroid is solely dependent on E2 and inhibited by P4 (46). However, recent studies concluded that P4 plays an equally important role as E2 in fibroid growth and maintenance (47,48). With the presence of E2, PRs will be expressed more in the fibroid cells to allow the binding of P4 to PRs. Once P4 binds to PR, the growth factors levels will be regulated to increase the expression of cell proliferation regulatory genes (PCNA, EGF, TGF-β3) and inhibits IGF-1 expression in the fibroid cells. Besides, P4 will stimulate the signalling pathways by increasing the proliferating cell nuclear antigen (causing proliferation) and anti-apoptotic B-cell lymphoma protein 2 while decreasing the cleaved caspase 3 (causing anti-apoptosis) (43,45). As a result, the fibroid cells will continue to proliferate without apoptosis (41,43).
Ishikawa et al. have studied on the types of hormone that is responsible for the fibroid growth and maintenance (44). They found that the fibroid which was treated with E2+P4 was significantly larger whereas fibroid treated with E2 or P4 alone was significantly reduced in volume. This showed that both E2 and P4 are mandatory for fibroid growth and maintenance, the absence of either one will not stimulate cellular proliferation. They also found high PR expression in any groups treated with E2, demonstrating the essential of E2 in PR upregulation.
Besides promoting fibroid growth, E2 and P4 are involved in uterine peristalsis. A study has demonstrated a significant higher peristalsis rate in E2 perfusion but lower in P4 perfusion (49). This is because the endometrial oxytocin and oxytocin receptor which are responsible UP are up-regulated by E2 (30). High E2 level stimulates the JZ and subsequently induces rapid uterine contraction whereas P4 antagonizes the effect of E2 and suppresses the uterine contractility.
In short, hormonal factors are involved in fibroid development. Medications which suppress E2 such as GnRH agonists, selective oestrogen receptor modulators and aromatase inhibitors and medications which suppress P4 like PR modulators will be beneficial in fibroid treatment.
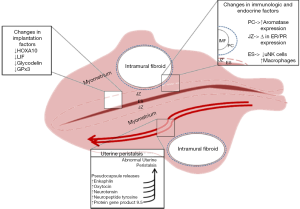
The summary of the causes of IM fibroids affect fertility are well described in Figure 2.
Does intramural (non-cavity distorting) fibroids cause infertility?
SM fibroids (type 1 and 2 fibroids) are proven to affect fertility and hysteroscopic resection-helps to improve the reproductive outcomes. SS fibroids (type 5, 6, 7 fibroids) do not affect fertility, as they do not protrude into the endometrial cavity (3). However, the effect of NCD IM fibroids such as type 3 and 4 fibroids on fertility remains controversial with studies yielding conflicting results.
Here, we will review on 10 retrospective studies and 5 prospective studies which show the impact of NCD IM fibroids on fertility outcomes. The participants of these studies consisted of women who were undergoing assisted reproductive treatment (ART) (IVF and ICSI) as they were believed to be more sensitive to study on the implantation process (50,51). The outcomes were determined based on the pregnancy rate (PR), clinical pregnancy rate (cPR), live birth rate (LBR), delivery rate (DR), implantation rate (IR) and miscarriage rate (MR).
Intramural fibroids reduce fertility outcomes
Few studies have suggested that NCD IM and SS fibroids cause significant adverse effects on the pregnancy outcomes (8,52-55).
Yan et al. reported significant reduction of cPR, biochemical pregnancy rate (bPR) and LBR among women with Type 3 fibroid compared to healthy controls (cPR =27.8% vs. 43.9%; bPR =29.1% vs. 51.4%; LBR =21.2% vs. 34.4%) (52). A single IM NCD fibroid with diameter <7 cm caused significant lower cPR and LBR compared to the control group (cPR =25.8% vs. 39.9%; LBR =17.7% vs. 30.9% respectively) (53). In Khalaf et al.’s study, the group with small IM fibroids demonstrated a significant reduction of cPR, ongoing PR (oPR) and LBR than the control group (cPR =23.6% vs. 32.9%; oPR =18.8% vs. 28.5%; LBR =14.8% vs. 24% respectively) (8). After adjusting the confounding variables, they found significant reduction of the oPR by 40% and LBR by 45% per cycle. Besides, Eldar-Geva et al. reported reduced PR in the SM (10%) and IM fibroids (16.4%) compared to SS fibroids (34.1%) and control (30.1%) (54). Although the cPR and oPR were significantly reduced in fibroid with diameter 22 mm in Hart et al.’s study, the results were not generalized as the women in the fibroids group were 2 years older than the control group (55).
Besides poor PR, the IR and MR were also affected by these fibroids (6,8,52-59). Yan et al. reported a significant reduction of IR in type 3 fibroids compared to the control group, 22.7% vs. 34.4% (52). However, there were no significant changes in the MR between both groups. Guven et al. also stated single NCD IM fibroid with diameter <7 cm caused significantly lower IR and non-significantly higher MR (53). In the study conducted by Surrey et al., the IR was significantly reduced among women <40 years old with fibroids compared to the age-matched control group, 21.4% vs. 33.3% respectively (57). However, no significant difference was found when comparing women >40 years old with fibroids and the age-matched control group, 17.5% vs. 11.6% respectively. Eldar-Geva et al. and Hart et al. also demonstrated significant reduction of IR among women with fibroids compared to the control group (54,55). A few studies reported insignificant increment of MR and reduction of the IR among women with fibroid compared to the control group (8,58,59). Somigliana et al. reported a higher IR in women with fibroids but it was due to the higher number of transferred embryos in women with fibroids (6).
Similar fertility outcome between study and control groups
In contrast to the above-mentioned studies, several studies demonstrated similar fertility outcomes in patients with and without fibroids indicating that the presence of NCD fibroids does not adversely affect the pregnancy outcomes (7,50,56,58-63). Vimercati et al. reported no significant differences in cPR, IM and MR between cavity distorting vs. NCD fibroids (56). Klatsky et al. also reported no statistical difference in cPR, IR and MR between patient with and without fibroids (cPR =47% vs. 54%, IR =36% vs. 38% and MR =15% vs. 9%) (61). Despite insignificantly lower PR in the IM and SS fibroid groups, Yarali et al. reported similar readings of cPR, IR, MR and multiple PR between groups of IM, SS and without fibroid (50). These results ran along with the study conducted by Oliveira et al. (7). Bozdag et al. reported comparable results of the bPR, cPR and IR between women with single IM fibroid and without fibroids (bPR =43% vs. 42%; cPR =36% vs. 38%; IR =20% vs. 19% respectively) (58).
No significant difference was found in the cPR, bPR, DR and MR when comparing women with fibroids with diameter <6 cm and women without fibroid (60,62). By comparing fibroids with diameter <4cm vs. control group, the cPR and IR were similar (7). Two prospective studies reported non-significant difference in the pregnancy outcomes between IM fibroids <5 cm with the control group except lower trend of LBR and DR, higher trend of MR in the fibroid groups (6,59). Aboulghar et al. reported non-significant differences of cPR between women with fibroids, with previous myomectomy and without fibroids (63). They also found that the PR in fibroids <5 mm away from the endometrial lining was lower but not significant when compared to fibroids >5 mm away from the endometrium lining.
Does size of IM fibroids affect fertility?
Several studies further analysed the effect of the fibroid size on the fertility outcomes (7,52,56,60). Yan et al. set the cutoff value of the fibroid diameter at 2 cm (type 3 fibroid) (52). The results showed a significant reduction of cPR, LBR, bPR and IR in fibroid diameter >2 cm compared to the control group. On the other hand, an earlier study conducted by the same authors showed NCD fibroids >2.85 cm had significant negative impact on the LBR and DR but insignificantly higher MR compared to the control group (60).
Christopoulas et al. reported multiple fibroids or NCD fibroids >3 cm lowered the cPR and LBR significantly (64). Vimercati et al. reported fibroids >4 cm have adverse impact in the pregnancy outcomes since they require more ART cycles (56). This statement was supported by another study conducted by Oliveira et al where IM fibroids >4 cm lowered the cPR and IR more than the IM <4 cm, SS fibroids and the control groups (7).
Correlation between the number and location of fibroids ith the fertility outcomes
Yan et al. reported absence of significant differences of cPR and DR on the number of fibroids as compared to the control group (60). They also found that single and multiple fibroids have the similar outcomes. No correlation was found between the number and location of the IM fibroids on the IVF-ICSI outcomes (6,7,50,59,61). Few studies reported absence of correlation between the size of the fibroids with cPR (6,50,58,61). Furthermore, Surrey et al. demonstrated no correlation between the total mean fibroid diameter or volume and the implantation among women with fibroids regardless of their age (57).
Review articles
There are two meta-analyses reporting on the effect of intramural fibroids on fertility. Sunkara et al. and Wang et al. reported statistically significant reduction of LBR and cPR (65,66). However, Sunkara et al. found no significant reduction in IM and no significant increase in MR among the study and control groups. Wang et al. demonstrated a significant reduction of IM and significant increase MR. Although Sunkara et al. have applied various strategy to increase the sensitivity and generality of their results, several limitations were difficult to be controlled (65). For example, different diagnostic tools were applied to confirm the normality of the uterine cavity, different inclusion and exclusion criteria as well as different mean size and number of the fibroids across the studies. On the other hand, Wang et al. was unable to match the age of the participants, number and mean size of the fibroids across the studies (66).
In summary, small IM fibroids (type 3 and 4) probably affect fertility and decrease PR and LBR in patients undergoing ART. It is difficult to decide which patients with small IM fibroids will benefit from surgical or non-surgical intervention.
Does removal of intramural fibroids (myomectomy) improve pregnancy rates?
Myomectomy improves pregnancy
Bulletti et al. reported higher PR and LBR post-laparoscopic myomectomy compared to non-surgical group (LBR =42% vs. 11% respectively) (67). They concluded that surgical removal of large and multiple fibroids resulted in better outcomes but there were also positive results for women with smaller fibroids. Campo et al. also reported significant improvement in the pregnancy outcomes post-myomectomy among women with SS or IM fibroids without undergoing ART (68). The DR was improved from 38.5% pre-surgery to 86.2% post-surgery while MR was reduced from 61.5% to 13.8% respectively. These improvements were resulted from the removal of the plausible cause of impaired fertility such as altered UP and blood supply.
Yoshino et al. identified patients with IM fibroid who have increased frequency of UP by cMRI (discussed earlier in the UP section under pathophysiology) (69). They then performed myomectomy on all these patients. MRI was done post-myomectomy to determine the reduction of UP and PR was evaluated following 8 months of ART. As a result, 14 of 15 patients had normalized UP, 6 of them achieved pregnancy. The authors also suggested that cMRI might play a role in selecting patient who is required for surgery.
Myomectomy according to fibroid size / diameter
In the earlier discussion, we have seen that not all studies with IM fibroids decrease fertility potential. As such the size of fibroid/s may be important in determining which fibroid/s may benefit from myomectomy (70). Yan et al. suggested that resection of type 3 fibroids with diameter >2 cm may improve fertility while Benecke et al. concluded that surgery will be beneficial for patients with history of unsuccessful pregnancy with IM fibroids >2 cm without other infertility factors (52,71). Kolankaya et al. reported that most surgeons recommend surgery for fibroids >7 cm or women with multiple failed IVF cycles (72). Vimercati et al. supported pre-IVF myomectomy for fibroids >4 cm (56). Bulletti et al. demonstrated higher success rate and DR among patients with IM fibroids >5 cm and underwent laparoscopic myomectomy prior to IVF (73). The PR was 33% and DR was 25% in the myomectomy group while 15% and 12% in the control group with no significant difference in the MR.
Myomectomy according to fibroid location
The fibroid location is important in determining the necessity of myomectomy. Casini et al. reported that the PR post-myomectomy was higher in all types of fibroids compared to non-surgical group (74). When there is a SM component of the fibroid, myomectomy showed a statistically significant improvement in PR. This was not seen in patients with IM and SS-IM fibroids but there was an overall higher trend of PR among these women who underwent myomectomy compared to those with fibroid left in situ. Besides, myomectomy can reduce MR in most of the fibroids and improve the chances of fertilization to achieve better pregnancy outcomes.
Myomectomy is not advisable
Myomectomy is associated with surgical morbidities (53,56,59,64). There are three studies which do not advocate myomectomy as a routine treatment for infertile women with fibroids. Aboulghar et al. demonstrated no significant difference in the cPR between the groups of surgical, non-surgical and infertile women without fibroids (36% vs. 29% vs. 36% respectively) (63). They also found no statistically significant in the cPR regardless of the distance between the fibroid and the endometrial lining except a trend of higher cPR when fibroids were at >5 mm away from the endometrial lining. Cochrane review also found no improvement in the fertility outcome post-myomectomy regardless of the fibroid location (75). Belina et al. against myomectomy in women with IM fibroids regardless of the size (76). They encouraged women to weigh the benefits of the surgery against the risks.
Some authors recommend surgery only for cases like repeated IVF treatment failure, fibroid related obstetrical complications and recurrent miscarriage (6,62). A review study concluded that there is no significant difference in the fertility outcomes between surgical and non-surgical groups (77). Although IM fibroids reduced fertility and increased MR, myomectomy did not significantly increase the cPR. They concluded that removal of intracavitary fibroid is beneficial, but removal of NCD IM fibroids does not significantly increase the cPR and LBR. Although NCD IM fibroids significantly decreased the LBR and cPR, it does not mean that removal of such fibroids will restore the LBR to the expected rate among women without fibroids (65).
Alternative to myomectomy (non-surgical methods)
Since myomectomy may be considered a big procedure for small fibroids and its benefit is still controversial, is it possible to consider non-surgical methods to improve fertility in such patients? Here we will look at some alternatives to myomectomy namely ulipristal acetate (UPA), gonadotrophin releasing hormone agonist (GnRHa), atosiban, uterine artery embolization (UAE) and high intensity focussed ultrasound (HIFU).
UPA
UPA is a selective progesterone receptor modulator (SPRM) which is a synthetic steroid that have agonistic and antagonistic effects on the PR (43,78,79). Since its structure is like P4, they will compete at the PR binding site. However, UPA has higher selectivity for PR than P4, so it is more potent to modulate the PR activity (41,43). This unique selectivity makes SPRM superior to GnRHa as it maintains the circulating E2 level within the mid-follicular phase range thereby avoiding the side effects of hypoestrogenism which are commonly reported in GnRHa treatment (43,78-82).
SPRM specifically acts on the pituitary gland, fibroid and endometrium (78,80). By acting on the hypothalamic-pituitary-ovarian axis, UPA reduces the secretion of luteinizing hormone (LH) and follicular-stimulating hormone (FSH) to induce amenorrhea and inhibit ovulation. Alongside with its action at the PR on the endometrium, menorrhagia can be controlled. In the fibroid cells, UPA acts as P4 antagonist to inhibit cellular proliferation and induce cellular apoptosis. Neovascularization can also be inhibited by reducing the angiogenic growth factors. To shrink fibroid, UPA increases matrix metalloproteinases and decreases tissue inhibitor thereby reduces the collagen deposition in the fibroid extracellular matrix (43,80,81).
Five case reports focused on the fertility outcome post-UPA without surgical treatment (41,81,83-85). Among these, 2 case reports demonstrated spontaneous pregnancy while the remaining 3 reported successful ART post-UPA. Besides proving a significant reduction of the fibroid size, they also showed sustained effect in maintaining the fibroid volume for up to 6 months after treatment cessation. UPA helps to increase the distance between the fibroid and the endometrium lining, subsequently restore the uterine anatomy to allow embryo implantation without interfering the endometrial receptivity (41,83). UPA is superior to myomectomy in terms of lower risks and complications with shorter waiting period to conceive. The optimal waiting period for conception post-myomectomy was 9.8 months and majority of the pregnancies occur within 2 years to allow wound healing and better uterine perfusion. This long waiting period is insecure especially for advanced-age infertile patients. With UPA, patients can conceive immediately post-treatment (41,85). All these case reports are based on patients with type 2 or 3 fibroids. Whether this can be extrapolated to benefit type 4 NCD fibroids is yet to be confirmed in studies.
As UPA selectively acts on the PRs, the unopposed E2 stimulation causes endometrial overgrowing and thickening. This endometrial modification is known as PR modulator associated endometrial changes (PAEC) (43,78,80,86). It is a benign and reassuring condition as it is reversible after treatment cessation. It usually resolves within 6 months post-treatment where the endometrium thickness and quality will be restored for blastocyst implantation (81,87-89).
UPA has been reported to cause serious liver injury in a few patients (78). Few recommendations on prescribing UPA have been released by the Pharmacovigilance Risk Assessment Committee to maintain its safety profile.
Gonadotrophin releasing hormone agonist (GnRHa)
Gonadotrophin releasing hormone (GnRH) is a native peptide which regulates the production of FSH and LH to stimulate the ovaries to produce sex steroid hormone like 17β-estradiol and P4 (90).
GnRHa acts directly on the pituitary gland by binding to the GnRH receptors (91). Initially, it will increase the release of gonadotropins causing a surge of FSH and LH. After 1–3 weeks, the GnRH receptors will be desensitized and downregulates the hypothalamic-pituitary-ovarian axis, causing a reduction in E2 and P4 thus causing a reduction in fibroid growth (92). GnRHa also acts on the GnRH receptors expressed by the fibroids to reduce cellular proliferation (91). Besides decreasing the expression of several growth factors, it also reduces the expression of the nuclear factor of activated T cells 5 which acts as a hyperosmolarity gene (90,91). During this process, the water will diffuse out from the fibroid cells causing shrinkage of the fibroid.
Few studies have been conducted to study the effect of GnRHa on fibroid volume reduction. They have proven a significant reduction of the fibroid volume during the treatment period (93-95). One of the review articles stated that five studies reported 30–60% reduction of the fibroid size (96-99). Friedman et al. demonstrated a 6-month treatment of 3.75 mg leuprolide acetate caused 36% reduction of uterine volume at 12 weeks while 45% at 24 weeks of treatment (100). Since there are lack of studies to determine the effect of GnRHa on fertility, we postulate that the significant reduction of fibroid volume by GnRHa may reduce the impact of cavity encroaching fibroid and subsequently improve implantation. In the study conducted by Kessel et al., 3 out of 5 infertile women with fibroids successfully conceived, 2 of them conceived without surgical intervention (95).
Atosiban
Atosiban is a combined oxytocin and vasopressin V1a receptor antagonist. As an oxytocin receptor antagonist, atosiban competes with oxytocin at the oxytocin receptors in the endometrial cells to decrease endometrial contraction and prevent embryo expulsion during the implantation phase (101,102). By reducing the oxytocin effect, it will inhibit the oxytocin-induced prostaglandin production and increase the endometrial blood supply (101-105). As a vasopressin V1A antagonist, atosiban relaxes the uterine arteries and decreases the systolic blood pressure to improve blood perfusion to the endometrium and myometrium (101-103). These antagonistic effects on the oxytocin and vasopressin receptors improve uterine receptivity and embryo implantation.
Atosiban is uterine specific which has immediate and profound effect on the uterine activity (103,106,107). Since UP is one of the suspected causes of infertility in patients with IM fibroid, atosiban may be used to improve PR in such patients.
However, all studies done with atosiban recruited patients with repeated implantation failures. Four studies have proven significant increase in the cPR, LBR and IR and significant decrease in MR in atosiban group compared to the placebo group (101,102,105,106). Two case reports have demonstrated positive pregnancy outcomes after receiving atosiban (103,107). These studies have also shown a significant reduction of uterine contractions post-atosiban (103,105,107). However, double-blinded RCT showed non-significant changes in the LBR post-atosiban to women with repeated implantation failure including women with fibroids (104). Whether atosiban will improve PR in patients with NCD IM fibroid is yet to be determined in studies.
UAE
UAE is performed by injecting small embolic particles into both uterine arteries to occlude the target vessel. This will induce ischemia leading to necrosis of the fibroid cells (108). While obstructing the blood supply to the fibroids, the vascularity of the whole endometrium and myometrium will be affected during UAE (109,110). UAE is believed to affect embryonic implantation and difficulties in maintaining gestation leading to increased miscarriages. The risk of amenorrhoea and ovarian failure after UAE in young women is low. However, there is a worry of poor oocyte quality and poor response to ovarian stimulation in patients who have under gone UAE (111-113). Two patients <40 years old who underwent IVF post-UAE showed low response to ovarian stimulation (114). Four patients desiring pregnancy became amenorrheic post-UAE and another patient underwent unsuccessful IVF treatment (115). Several cases of intrauterine adhesions, endometrial atrophy and fistula between the uterine cavity and the embolized myoma post-UAE were described in the literature (115-117). >1/3 of 127 patients were found to have intracavity tissue necrosis 3–9 months post-UAE (118). This alarming finding could help to explain the high MR in post-UAE women.
Therefore, UAE is listed as a relative contraindication for women desiring future fertility by the American College of Obstetrics and Gynaecology, the Society of Interventional Radiology and the Royal College of Obstetrics and Gynecology (119-121). However, there are several studies reporting pregnancies after UAE. Karisen et al. reviewed articles and concluded that 50% of the women achieved pregnancy post-UAE, which is lower than post-myomectomy (78%) (122). MR appears to be higher post-UAE (60%) than post-myomectomy (20%).
As the benefits of UAE on fertility remains debatable and a low level of evidence to suggest better pregnancy outcomes post-UAE, some authors advice UAE should not be the 1st choice for women with fibroids and have future pregnancy plans (108,122,123).
HIFU
HIFU is also known as focused ultrasound surgery. It is an organ sparing, non-invasive, thermal ablative procedure. It uses an extracorporeal transducer to focus the high-intensity ultrasonic beams to the targeted myoma to thermally ablate the tumours without introducing needles or probes into the tumour (124,125). There are two types of HIFU treatment namely Magnetic Resonance Imaging focused ultrasound surgery (MRgFUS) and Ultrasound guided high intensity focused ultrasound (USgHIFU). HIFU treatment is limited to ablation within the pseudomembrane. Therefore, there is minimal damage to the surrounding normal myometrium without obvious damage to the elastic and collagen fibers in the normal uterine muscle, resulting in less scar tissue formation and less risk of collagen fiber hyperplasia. Theoretically, this would reduce the pregnancy risks in women who have undergone HIFU treatment for uterine fibroids, as compared to myomectomy. Previous studies have found that, compared with laparoscopic myomectomy, HIFU has the advantages of fewer complications, faster recovery, less patient discomfort, and lower treatment-associated risk (126,127). Clinical studies also confirmed that HIFU avoids ovarian function impairment and adverse reaction, thus preserve the ability to conceive (128-132).
Li et al. found that the volume of the uterine fibroids decreased, and the symptoms of uterine fibroids improve significantly post-HIFU treatment (133). The PR post-HIFU reached 69.3% (despite not considering male infertility), which is similar to the PR (62.2–68%) post-myomectomy (134-136). Almost 74% of women conceived within one-year post-HIFU treatment. The spontaneous PR post-HIFU was 95.4%, which is slightly higher than that of post-myomectomy (64.6–88.6%) (134,137). Although the spontaneous abortion rate (14.9%) post-HIFU is similar to that of post-myomectomy (13–24%), this rate is still significantly lower than in pregnancy with untreated fibroids (20–46.7%) (138,139).
Discussion
NCD IM fibroid with infertility poses a difficult clinical challenge. There appears to be enough evidence that NCD IM fibroid affects fertility. Type 3 fibroids may have a higher risk of poor pregnancy outcome compared to type 4 fibroids (52). Since disruption of the JZ appears to be an important cause of subfertility, type 4a fibroids may have a poorer outcome than type 4b fibroids. This will be difficult to prove in clinical studies because it is difficult to visualize the JZ and the changes in its thickness during the menstrual cycle on transvaginal ultrasound.
There are many possible causes why IM fibroids affect fertility. The only measurable cause appears to be increased UP. Unfortunately, not all patients with IM fibroids have increased UP. Currently there is no good and inexpensive method of measuring UP. cMRI seems to be an accurate method but it is expensive (30). Transvaginal ultrasound method is a cheaper modality but it is still very user dependent. A better method needs to be devised to measure UP easily and effectively.
With vitrification, pregnancy after frozen embryo transfer is improving (140). As such, it has been suggested that myomectomy should not be the first line treatment in patients with small IM fibroids. In patients with many frozen embryos, the strategy could be to consider surgical or non-surgical intervention only in patients who have a failed embryo transfer.
Myomectomy may appear to be a big procedure to perform in patients with small IM fibroid/s. Non-surgical modalities described above may be another option. While UPA seems encouraging, this may be an option to reduce the size of fibroids especially the type 3 and 4a fibroids. The shrinkage may move the fibroid away from the endometrial lining and the JZ thus improving IR. However, this needs to be balanced with the risk of liver complications. GnRHa may have similar effect as UPA although this has not been researched in clinical studies. In patients with increased UP, atosiban may be given but this option has not been explored in clinical studies as well. UAE may not be a good option for small IM fibroids because of the complications discussed above. HIFU is an attractive option to shrink these small IM fibroids because it is a non-invasive technique with very few side effects. Its use in small fibroids has not been explored in clinical studies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm.2019.11.01/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100-7. [Crossref] [PubMed]
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 1981;36:433-45. [Crossref] [PubMed]
- Lasmar RB, Barrozo PR, Dias R, et al. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment - preliminary report. J Minim Invasive Gynecol 2005;12:308-11. [Crossref] [PubMed]
- Stamatellos I, Bontis J. Hysteroscopic myomectomy. Eur Clin Obstet Gynecol 2007;3:17-23. </jrn>. [Crossref]
- Munro MG, Critchley HO, Fraser IS. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet 2018;143:393-408. [Crossref] [PubMed]
- Somigliana E, De Benedictis S, Vercellini P, et al. Fibroids not encroaching the endometrial cavity and IVF success rate: a prospective study. Hum Reprod 2011;26:834-9. [Crossref] [PubMed]
- Oliveira FG, Abdelmassih VG, Diamond MP, et al. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization - intracytoplasmic sperm injection. Fertil Steril 2004;81:582-7. [Crossref] [PubMed]
- Khalaf Y, Ross C. The effect of small intramural uterine fibroids on the cumulative outcome of assisted conception. Hum Reprod 2006;21:2640-4. [Crossref] [PubMed]
- Pier BD, Bates GW. Potential causes of subfertility in patients with intramural fibroids. Fertil Res Pract 2015;1:12. [Crossref] [PubMed]
- Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update 2011;17:242-53. [Crossref] [PubMed]
- Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril 2010;93:2027-34. [Crossref] [PubMed]
- Matsuzaki S, Canis M, Darcha C, et al. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod 2009;24:3180-7. [Crossref] [PubMed]
- Alizadeh Z, Faramarzi S, Saidijam M, et al. Effect of intramural myomectomy on endometrial HOXA 10 and HOXA 11 mRNA expression at the time of implantation window. Iran J Reprod Med 2013;11:983-8. [PubMed]
- Seppälä M, Koistinen H, Koistinen R, et al. Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations. Hum Reprod Update 2007;13:275-87. [Crossref] [PubMed]
- Ben-Nagi J, Miell J, Mavrelos D, et al. Endometrial implantation factors in women with submucous uterine fibroids. Reprod Biomed Online 2010;21:610-5. [Crossref] [PubMed]
- Richlin SS, Ramachandran S, Shanti A, et al. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Hum Reprod 2002;17:2742-7. [Crossref] [PubMed]
- Farimani Sanoee M, Alizamir T, Faramarzi S, et al. Effect of Myomectomy on Endometrial Glutathione Peroxidase 3 (GPx3) and Glycodelin mRNA Expression at the Time of the Implantation Window. Iran Biomed J 2014;18:60-6. [PubMed]
- Brosens I, Derwig I, Brosens J, et al. The enigmatic uterine junctional zone: The missing link between reproductive disorders and major obstetrical disorders? Hum Reprod 2010;25:569-74. [Crossref] [PubMed]
- Ciavattini A, Di Giuseppe J, Stortoni P, et al. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int 2013;2013:173184 [Crossref] [PubMed]
- Hricak H, Alpers C, Crooks LE, et al. Magnetic resonance imaging of the female pelvis: initial experience. AJR Am J Roentgenol 1983;141:1119-28. [Crossref] [PubMed]
- Jakimiuk AJ, Bogusiewicz M, Tarkowski R, et al. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertil Steril 2004;82:1244-9. [Crossref] [PubMed]
- Ishikawa H, Reierstad S, Demura M, et al. High Aromatase Expression in Uterine Leiomyoma Tissues of African-American Women. J Clin Endocrinol Metab 2009;94:1752-6. [Crossref] [PubMed]
- Kitaya K, Yasuo T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum Immunol 2010;71:158-63. [Crossref] [PubMed]
- Togashi K, Kawakami S, Kimura I, et al. Uterine contractions: possible diagnostic pitfall at MR imaging. J Magn Reson Imaging 1993;3:889-893. [Crossref] [PubMed]
- Fujiwara T, Togashi K, Yamaoka T, et al. Kinematics of the Uterus: Cine Mode MR Imaging. Radiographics 2004;24:e19 [Crossref] [PubMed]
- Lyons EA, Taylor PJ, Zheng XH, et al. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil Steril 1991;55:771-4. [Crossref] [PubMed]
- Kido A, Ascher SM, Hahn W, et al. 3 T MRI uterine peristalsis: comparison of symptomatic fibroid patients versus controls. Clin Radiol 2014;69:468-72. [Crossref] [PubMed]
- Orisaka M, Kurokawa T, Shukunami K, et al. A comparison of uterine peristalsis in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol 2007;135:111-5. [Crossref] [PubMed]
- Yoshino O, Hori M, Osuga Y, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum Reprod 2010;25:2475-9. [Crossref] [PubMed]
- Kuijsters NP, Methorst WG, Kortenhorst MSQ, et al. Uterine peristalsis and fertility: current knowledge and future perspectives: a review and meta-analysis. Reprod Biomed Online 2017;35:50-71. [Crossref] [PubMed]
- Malvasi A, Cavallotti C, Morroni M, et al. Uterine fibroid pseudocapsule studied by transmission electron microscopy. Eur J Obstet Gynecol Reprod Biol 2012;162:187-91. [Crossref] [PubMed]
- De Falco M, Staibano S, Mascolo M, et al. Leiomyoma pseudocapsule after pre-surgical treatment with gonadotropin-releasing hormone agonists: relationship between clinical features and immunohistochemical changes. Eur J Obstet Gynecol Reprod Biol 2009;144:44-7. [Crossref] [PubMed]
- Di Tommaso S, Massari A, Bozzetti MP, et al. Gene expression analysis reveals an angiogenic profile in uterine leiomyoma pseudocapsule. Mol Hum Reprod 2013;19:380-7. [Crossref] [PubMed]
- Tinelli A, Mynbaev OA, Mettler L, et al. A combined ultrasound and histologic approach for analysis of uterine fibroid pseudocapsule thickness. Reprod Sci 2014;21:1177-86. [Crossref] [PubMed]
- Malvasi A, Cavallotti C, Nicolardi G, et al. NT, NPY and PGP 9.5 presence in myomeytrium and in fibroid pseudocapsule and their possible impact on muscular physiology. Gynecol Endocrinol 2013;29:177-81. [Crossref] [PubMed]
- Shavell VI, Thakur M, Sawant A, et al. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil Steril 2012;97:107-10. [Crossref] [PubMed]
- Tinelli A, Malvasi A, Hurst BS, et al. Surgical management of neurovascular bundle in uterine fibroid pseudocapsule. Journal of the Society of Laparoendoscopic Surgeons 2012;16:119-29. [Crossref] [PubMed]
- Tinelli A, Mynbaev OA, Sparic R, et al. Physiology and importance of the myoma's pseudocapsule. Springer Online 2017.
- Tinelli A. Uterine fibroid pseudocapsule: an update of its importance in fibroid management and female reproduction. International Journal of Gynecological, Obstetrical and Reproductive Medicine Research 2014;1:7-10. </jrn>.
- Terry KL, De Vivo I, Hankinson SE, et al. Reproductive characteristics and risk of uterine leiomyoma. Fertil Steril 2010;94:2703-7. [Crossref] [PubMed]
- Kale AR. Ulipristal acetate for fibroids – IVF outcomes following treatment with UPA after IVF failure: series of 2 case reports. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2017;6:3177-81. </jrn>. [Crossref]
- McWilliams MM, Chennathukuzhi VM. Recent Advances in Uterine Fibroid Etiology. Semin Reprod Med 2017;35:181-9. [Crossref] [PubMed]
- Ali M, Al-Hendy A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biology of Reproduction 2017;97:337-52. [Crossref] [PubMed]
- Ishikawa H, Ishi K, Serna VA, Kakazu R, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010;151:2433-42. [Crossref] [PubMed]
- Botia CP, Camarasa SC, Baixauli FR, et al. Uterine fibroids: understanding their origins to better understand their future treatments. J Tumor Res 2017;3:3. </jrn>.
- Burroughs KD, Fuchs-Young R, Davis B, et al. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod 2000;63:1322-30. [Crossref] [PubMed]
- Lamminen S, Rantala I, Helin H, et al. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecol Obstet Invest 1992;34:111-4. [Crossref] [PubMed]
- Kawaguchi K, Fujii S, Konishi I, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol 1989;160:637-41. [Crossref] [PubMed]
- Mueller A, Siemer J, Schreiner S, et al. Role of estrogen and progesterone in the regulation of uterine peristalsis: results from perfused non-pregnant swine uteri. Hum Reprod 2006;21:1863-8. [Crossref] [PubMed]
- Yarali H, Bukulmez O. The effect of intramural and subserosal uterine fibroids on implantation rate and clinical pregnancy rate in patients having ICSI. Arch Gynecol Obstet 2002;266:30-3. [Crossref] [PubMed]
- Ng EH, Chan CC, Tang OS, et al. Endometrial and subendometrial blood flow measured by 3D power Doppler USS in patients with small intramural fibroids during IVF. Hum Reprod 2005;20:501-6. [Crossref] [PubMed]
- Yan L, Yu Q, Zhang YN, et al. Effect of type 3 intramural fibroids on in vitro fertilization-intracytoplasmic sperm injection outcomes: a retrospective cohort study. Fertil Steril 2018;109:817-822.e2. [Crossref] [PubMed]
- Guven S, Kart C, Unsal MA, et al. Intramural leoimyoma without endometrial cavity distortion may negatively affect the ICSI - ET outcome. Reprod Biol Endocrinol 2013;11:102. [Crossref] [PubMed]
- Eldar-Geva T, Meagher S, Healy DL, et al. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril 1998;70:687-91. [Crossref] [PubMed]
- Hart R, Khalaf Y, Yeong CT, et al. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum Reprod 2001;16:2411-7. [Crossref] [PubMed]
- Vimercati A, Scioscia M, Lorusso F, et al. Do uterine fibroids affect IVF outcomes? Reprod Biomed Online 2007;15:686-91. [Crossref] [PubMed]
- Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril 2001;75:405-10. [Crossref] [PubMed]
- Bozdag G, Esinler I, Boynukalin K, et al. Single intramural leiomyoma with normal hysteroscopic findings does not affect ICSI-embryo transfer outcome. Reprod Biomed Online 2009;19:276-80. [Crossref] [PubMed]
- Check JH, Choe JK, Lee G, et al. The effect on IVF outcome of small intramural fibroids not compressing the uterine cavity as determined by a prospective matched control study. Hum Reprod 2002;17:1244-8. [Crossref] [PubMed]
- Yan L, Ding L, Li C, et al. Effect of fibroids not distorting the endometrial cavity on the outcome of in vitro fertilization treatment: a retrospective cohort study. Fertil Steril 2014;101:716-21. [Crossref] [PubMed]
- Klatsky PC, Lane DE, Ryan IP, et al. The effect of fibroids without cavity involvement on ART outcomes independent of ovarian age. Hum Reprod 2007;22:521-6. [Crossref] [PubMed]
- Nejad EST, Moini A, Amirchaghmaghi E, et al. Effect of IM uterine myoma on the outcome of ART cycles. Iranian Journal of Reproductive Medicine 2007;5:65-8. </jrn>.
- Aboulghar MM, Al-Inany HG, Aboulghar MA, et al. The effect of IM fibroids on the outcome of IVF. Middle East Fertility Society Journal 2004;9:263-7. </jrn>.
- Christopoulos G, Vismas A, Salim R, et al. Fibroids that do not distort the uterine cavity and IVF success rates: an observational study using extensive matching criteria. BJOG 2017;124:615-21. [Crossref] [PubMed]
- Sunkara SK, Khairy M. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod 2010;25:418-29. [Crossref] [PubMed]
- Wang X, Chen L, Wang H, et al. The Impact of Noncavity-Distorting Intramural Fibroids on the Efficacy of In Vitro Fertilization-Embryo Transfer: An Updated Meta-Analysis. Biomed Res Int 2018;2018:8924703 [PubMed]
- Bulletti C, Ziegler DD, Polli V, et al. The role of leiomyomas in infertility. J Am Assoc Gynecol Laparosc 1999;6:441-5. [Crossref] [PubMed]
- Campo S, Campo V, Gambadauro P. Reproductive outcome before and after laparoscopic or abdominal myomectomy for subserous or intramural myomas. Eur J Obstet Gynecol Reprod Biol 2003;110:215-9. [Crossref] [PubMed]
- Yoshino O, Nishii O, Osuga Y, et al. Myomectomy decreases abnormal uterine peristalsis and increase pregnancy rate. J Minim Invasive Gynecol 2012;19:63-7. [Crossref] [PubMed]
- Ben-Rafael Z. Should we operate on fibroids before IVF? Expert Review of Obstetrics & Gynecology 2013;8:205-11. </jrn>. [Crossref]
- Benecke C, Kruger TF, Siebert TI, et al. Effect of fibroids on fertility in patients undergoing assisted reproduction: a structured literature review. Gynecol Obstet Invest 2005;59:225-30. [Crossref] [PubMed]
- Kolankaya A, Arici A. Myomas and assisted reproductive technologies: when and how to act? Obstet Gynecol Clin North Am 2006;33:145-52. [Crossref] [PubMed]
- Bulletti C. Myomas, pregnancy outcomes, and in vitro fertilization. Ann N Y Acad Sci 2004;1034:84-92. [Crossref] [PubMed]
- Casini ML, Rossi F, Agostini R, et al. Effects of position of fibroids on fertility. Gynecol Endocrinol 2006;22:106-9. [Crossref] [PubMed]
- Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev 2012;11:CD003857 [PubMed]
- Carranza-Mamane B, Havelock J, et al. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can 2015;37:277-85. [Crossref] [PubMed]
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systemic review of evidence. Fertil Steril 2009;91:1215-23. [Crossref] [PubMed]
- Rabe T, Saenger N, Elbert AD, et al. Selective Progesterone Receptor Modulators for the Medical Treatment of Uterine Fibroids with a Focus on Ulipristal Acetate. Biomed Res Int 2018;2018:1374821 [PubMed]
- Piecak K, Milart P, Wozniakowska E, et al. Ulipristal acetate as a treatment option for uterine fibroids. Prz Menopauzalny 2017;16:133-6. [Crossref] [PubMed]
- Biglia N, Carinelli S, Maiorana A, et al. Uipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther 2014;8:285-92. [PubMed]
- Murad K. Spontaneous pregnancy following ulipristal acetate treatment in a woman with symptomatic uterine fibroid. J Obstet Gynaecol Can 2016;38:75-9. [Crossref] [PubMed]
- de la Fuente E, Borrás MD, Rubio M, et al. Ulipristal acetate in myomectomy optimization in an infertile patient with giant myomas. Case Rep Med 2016;2016:5135780 [Crossref] [PubMed]
- Monleón J, Martinez-Varea A, Galliano D, et al. Successful Pregnancy after Treatment with Ulipristal Acetate for Uterine Fibroids. Case Rep Obstet Gynecol 2014;2014:314587 [Crossref] [PubMed]
- Wdowiak A. Pre-treatment with ulipristal acetate before ICSI procedure: A case report. Menopausal Review 2013;6:496-500. </jrn>. [Crossref]
- Orvieto R, Zilberberg E, Vanni VS, et al. A novel approach to infertility treatment of advance-age patient with prominent intramural fibroid. Gynecol Endocrinol 2018;34:551-3. [Crossref] [PubMed]
- Williams AR, Bergeron C, Barlow DH, et al. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator. Int J Gynecol Pathol 2012;31:556-69. [Crossref] [PubMed]
- Luyckx M, Squifflet JL, Jadoul P, et al. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 2014;102:1404-9. [Crossref] [PubMed]
- Lo Monte G, Piva L, Graziano A, et al. Ulipristal acetate prior to in vitro fertilization in a female patient affected by uterine fibroids: a case report. Eur Rev Med Pharmacol Sci 2016;20:202-7. [PubMed]
- Levy G, Avilia N, Amstrong A, et al. Does the selective progesterone receptor modulator ulipristal normalize the uterine cavity in women with leiomyoma? J Fertiliz In Vitro 2011. DOI:
10.4172/2165-7491.1000102 - Lewis TD, Malik M, Britten J, et al. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. Biomed Res Int 2018;2018:2414609 [Crossref] [PubMed]
- Hodgson R, Bhave Chittawar P, Farquhar C. GnRH agonists for uterine fibroids (Protocol). Cochrane Database Syst Rev 2017;10. </jrn>.
- Sohn GS, Cho SH, Kim YM, et al. Current medical treatment of uterine fibroids. Obstet Gynecol Sci 2018;61:192-201. [Crossref] [PubMed]
- Minaguchi H, Wong JM, Snabes MC. Clinical use of nafarelin in the treatment of leiomyomas. A review of the literature. J Reprod Med 2000;45:481-9. [PubMed]
- Broekmans FJ, Hompes PG, Heitbrink MA, et al. Two-step gonadotropin-releasing hormone agonist treatment of uterine leiomyomas: standard-dose therapy followed by reduced-dose therapy. Am J Obstet Gynecol 1996;175:1208-16. [Crossref] [PubMed]
- Kessel B, Liu J, Mortola J, et al. Treatment of uterine fibroids with agonist analogs of gonadotropin-releasing hormone. Fertil Steril 1988;49:538-541. [Crossref] [PubMed]
- Olive DL, Lindheim SR, Pritts EA. Non-surgical management of leiomyoma: Impact on fertility. Curr Opin Obstet Gynecol 2004;16:239-43. [Crossref] [PubMed]
- Friedman AJ, Harrison-Atlas D, Barbieri RL, et al. A randomized, placebo-controlled, double-blind study evaluating the efficacy of leuprolide acetate depot in the treatment of uterine leiomyomata. Fertil Steril 1989;51:251-6. [Crossref] [PubMed]
- Schlaff WD, Zerhouni EA, Huth JA, et al. Placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (Leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol 1989;74:856-62. [PubMed]
- Friedman AJ, Rein MS, Harrison-Atlas D, et al. A randomized, placebo-controlled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril 1989;52:728-33. [Crossref] [PubMed]
- Friedman AJ, Hoffman DI, Comite F, et al. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The leuprolide study group. Obstet Gynecol 1991;77:720-5. [PubMed]
- Moraloglu O, Tonguc E, Var T, et al. Treatment with oxytocin antagonists before embryo transfer may increase implantation rates after IVF. Reprod Biomed Online 2010;21:338-43. [Crossref] [PubMed]
- Mishra V, Agarwal H, Goel S, et al. A prospective case control trial to evaluate and compare the efficacy and safety of atosiban vs placebo in IVF-ET program. J Hum Reprod Sci 2018;11:155-60. [Crossref] [PubMed]
- Pierzynski P, Reinheimer TM, Kuczynski W. Oxytocin antagonists may improve infertility treatment. Fertil Steril 2007;88:213.e19-22. [Crossref] [PubMed]
- Ng EH, Li RH, Chen L, et al. A randomized double blind comparison of atosiban in patients undergoing IVF treatment. Hum Reprod 2014;29:2687-94. [Crossref] [PubMed]
- Lan VT, Khang VN, Nhu GH, et al. Atosiban improves implantation and pregnancy rates in patients with repeated implantation failure. Reprod Biomed Online 2012;25:254-60. [Crossref] [PubMed]
- Chou PY, Wu MH, Pan HA, et al. Use of an oxytocin antagonist in in vitro fertilization-embryo transfer for women with repeated implantation failure: a retrospective study. Taiwan J Obstet Gynecol 2011;50:136-40. [Crossref] [PubMed]
- Liang YL, Kuo TC, Hung KH, et al. Oxytocin antagonist for repeated implantation failure and delay of delivery. Taiwan J Obstet Gynecol 2009;48:314-6. [Crossref] [PubMed]
- Czuczwar P, Stepniak A, Wrona W, et al. The influence of uterine artery embolization on ovarian reserve, fertility and pregnancy outcomes – a review of literature. Prz Menopauzalny 2016;15:205-9. [Crossref] [PubMed]
- Redecha M Jr, Mizickva M, Javorka V, et al. Pregnancy after uterine artery embolization for the treatment of myomas: a case series. Arch Gynecol Obstet 2013;287:71-6. [Crossref] [PubMed]
- Bonduki CE, Feldner PC, Silva JD Jr, et al. Pregnancy after uterine arterial embolization. Clinics (Sao Paulo) 2011;66:807-10. [Crossref] [PubMed]
- Kaump GR, Spies JB. The impact of uterine artery embolization on ovarian function. J Vasc Interv Radiol 2013;24:459-67. [Crossref] [PubMed]
- Spies JB, Roth AR, Gonsalves SM, et al. Ovarian function after uterine artery embolization for leiomyomata: assessment with use of serum follicle stimulating hormone assay. J Vasc Interv Radiol 2001;12:437-42. [Crossref] [PubMed]
- Tropeano G, Di Stasi C, Litwick K, et al. Uterine artery embolization for fibroids does not have adverse effects on ovarian reserve in regularly cycling women younger than 40 years. Fertil Steril 2004;81:1055-61. [Crossref] [PubMed]
- Mára M, Maskova J, Fucikova Z, et al. Remarks on embolization of uterine fibroids. Ceska Gynekol 2007;72:58-64. [PubMed]
- Torre A, Paillusson B, Fain V, et al. Uterine artery embolization for severe symptomatic fibroids: effects on fertility and symptoms. Hum Reprod 2014;29:490-501. [Crossref] [PubMed]
- Ogliari KS, Mohallem SV, Barrozo P, et al. A uterine cavity-myoma communication after uterine artery embolization: two case reports. Fertil Steril 2005;83:220-2. [Crossref] [PubMed]
- Tropeano G, Litwicka K, Di Stasi C, et al. Permanent amenorrhea associated with endometrial atrophy after uterine artery embolization for symptomatic uterine fibroids. Fertil Steril 2003;79:132-5. [Crossref] [PubMed]
- Mara M, Horak P, Kubinova K, et al. Hysteroscopy after uterine fibroid embolization: evaluation of intrauterine findings in 127 patients. J Obstet Gynaecol Res 2012;38:823-31. [Crossref] [PubMed]
- Royal College of Obstetrics & Gynaecology. Clinical recommendations on the use of uterine artery embolisation (UAE) in the management of fibroids. RCOG 2013.
- American College of Obstetricians and Gynaecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol 2008;112:387-400. [Crossref] [PubMed]
- Stokes LS, Wallace MJ, Godwin RB, et al. Quality improvement guidelines for uterine artery embolization for symptomatic leiomyomas. J Vasc Interv Radiol 2010;21:1153-63. [Crossref] [PubMed]
- Karlsen K, Hrobjartsson A, Korsholm M, et al. Fertility after uterine artery embolization of fibroids: a systemic review. Arch Gynecol Obstet 2018;297:13-25. [Crossref] [PubMed]
- Freed MM, Speis JB. Uterine artery embolization for fibroids: a review of current outcomes. Semin Reprod Med 2010;28:235-41. [Crossref] [PubMed]
- Mahmoud MZ, Alkhorayef M, Alzimami KS, et al. High-intensity focused ultrasound (HIFU) in uterine fibroid treatment: review study. Pol J Radiol 2014;79:384-90. [Crossref] [PubMed]
- Kim HK, Kim D, Lee MK, et al. Three cases of complications after high-intensity focused ultrasound treatment in unmarried women. Obstet Gynecol Sci 2015;58:542-6. [Crossref] [PubMed]
- Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Curr Opin Obstet Gynecol 2014;26:151-61. [Crossref] [PubMed]
- Bohlmann MK, Hoellen F, Hunold P, et al. High-Intensity Focused Ultrasound Ablation of Uterine Fibroids - Potential Impact on Fertility and Pregnancy Outcome. Geburtshilfe Frauenheilkd 2014;74:139-45. [Crossref] [PubMed]
- Qin J, Chen JY, Zhao WP, et al. Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet 2012;117:273-7. [Crossref] [PubMed]
- Cheung VY, Lam TP, Jenkins CR, et al. Ovarian Reserve After Ultrasound-Guided High-Intensity Focused Ultrasound for Uterine Fibroids: Preliminary Experience. J. Obstet Gynaecol Can 2016;38:357-61. [Crossref] [PubMed]
- Rabinovici J, David M, Fukunishi H, et al. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 2010;93:199-209. [Crossref] [PubMed]
- Pron G. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MRgHIFU) Treatment of Symptomatic Uterine Fibroids: An Evidence-Based Analysis. Ont Health Technol Assess Ser 2015;15:1-86. [PubMed]
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: A review of 9988 cases. Ultrason Sonochem 2015;27:671-6. [Crossref] [PubMed]
- Li JS, Wang Y, Chen JY, et al. Pregnancy outcomes in nulliparous women after ultrasound ablation of uterine fibroids: a single central retrospective study. Scientific Report, Chong Qing, China, 2017.
- Bernardi TS, Radosa MP, Weisheit A, et al. Laparoscopic myomectomy: a 6-year follow-up single-center cohort analysis of fertility and obstetric outcome measures. Arch Gynecol Obstet 2014;290:87-91. [Crossref] [PubMed]
- Fagherazzi S, Borgato S, Bertin M, et al. Pregnancy outcome after laparoscopic myomectomy. Clin Exp Obstet Gynecol 2014;41:375-9. [PubMed]
- Rossetti A, Sizzi O, Soranna L, et al. Fertility outcome: long-term results after laparoscopic myomectomy. Gynecol Endocrinol 2001;15:129-34. [Crossref] [PubMed]
- Koo YJ, Lee JK, Lee YK, et al. Pregnancy Outcomes and Risk Factors for Uterine Rupture After Laparoscopic Myomectomy: A Single-Center Experience and Literature Review. J Minim Invasive Gynecol 2015;22:1022-8. [Crossref] [PubMed]
- Radhika BH, Naik K, Shreelatha S, et al. Case series: Pregnancy Outcome in Patients with Uterine Fibroids. J Clin Diagn Res 2015;9:QR01-4. [PubMed]
- Klatsky PC, Tran ND, Caughey AB, et al. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol 2008;198:357-66. [Crossref] [PubMed]
- Shapiro BS, Daneshmand ST, Garner FC, et al. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril 2014;102:3-9. [Crossref] [PubMed]
Cite this article as: Supermaniam S, Thye WL. Intramural fibroid and fertility—to operate or not. Gynecol Pelvic Med 2019;2:31.


