
Robotic sentinel lymph node detection using indocyanine green
Introduction
The primary treatment of apparent early stage endometrial cancer (EC) is hysterectomy with bilateral salpingo-oophorectomy allowing the definition of disease on the basis of histology, grading, lympho-vascular space invasion (LVSI) and depth of myometrial invasion.
More than 90% of cases EC are diagnosed at early stage with a risk of nodal metastases <1% in “low-risk” population, according to Mayo Clinic criteria (grade 1 or 2, myometrial invasion <50%, and tumor diameter <2 cm) compared to a 16% for intermediate risk endometrioid adenocarcinoma (myometrial invasion ≥50%, and tumor diameter ≥2 cm), and 20–40% for high-risk cases (endometrioid grade 3, clear cell, serous, and carcinosarcoma) (1).
The role of retroperitoneal staging is controversial; therapeutic role of systematic lymphadenectomy in presumed early stage EC is still debated: two randomized clinical studies [ASTEC 2009 (2) and Benedetti Panici 2008 (3)] showed no therapeutic benefit to systematic lymphadenectomy; however SEPAL study 2010 (4), even though retrospective, demonstrated a significant improvement in overall survival in intermediate and high risk patients who had undergone systematic lymphadenectomy.
The increasing interest on sentinel lymph node (SLN) mapping in EC was lead to reduce morbidity of a full staging procedure, such us intra-operative complications (blood loss, vascular and nerve injury, conversion to laparotomy, operating time, length of stay), short and long term complications (lymphocysts, chylous ascites, lymphedema).
It is also not to be overlooked the possibility to identify metastases in low-risk patients and the increased detection rate of low volume metastases by ultra-staging.
SLN biopsy was first described for EC by Burke et al. (MD Anderson Cancer Center) in 1996 (5). Further single and multi - institution series described the feasibility and the high detection rate of the technique. The wide experience of Memorial Sloan Kettering Cancer Center contributed to develop the technique, introducing the SLN biopsy algorithm as part of routine surgical staging, bringing about the publication in 2017 of the SGO (Society of Gynecologic Oncology) Consensus recommendations, although there aren’t randomized controlled trial yet (6).
A meta-analysis published in 2017 (7) showed a pooled sensitivity of 96% and NPV of 99.7%.
Indocyanine green (ICG) was the most commonly used tracer in different series; cervical injection of this tracer has been shown (7) to result in significant higher bilateral pelvic detection rate compared with uterine injection (75% vs. 51%), thereby avoiding a technically complex hysteroscopic peritumoral or transabdominal fundal injection. In 2017 FIRES study (8), a multicentre, prospective, cohort study showed that, in early stage EC of all histologies, SLN identified with ICG has a sensitivity of 97.2% (95% CI: 85–100), and a negative predictive value of 99.6% (95% CI: 97.9–100), suggesting that such a procedure can safely replace systematic lymphadenectomy.
Operative technique
The tracer ICG is a sterile lyophilized green powder; it is a water soluble, tricarbocyanine dye with peak spectral absorption at 800 nm. Following intravenous injection, ICG is mostly carried by albumin, is pick up by the hepatic cells and secreted into the bile. Based on these characteristics ICG has become indicated in determining cardiac output, hepatic function, liver blood flow, and in ophthalmic angiography. The use of this drug in lymphatic mapping is still off-label.
ICG contains sodium iodide and should be used with caution in patients who have a history of allergy to iodides.
Near-infrared imagers are filtered to receive the wavelength emitted by ICG and are available for laparotomy, laparoscopy, and robotic surgery; for robotic surgery it is available on the da Vinci Si or Xi surgical robot platforms (Intuitive Surgery, Sunnyvale, CA, USA) with the Firefly fluorescence imaging technology.
The Firefly mode, activated at the surgeon console, allows to navigate with real-time, fluorescence-guided identification of lymphatic channels and SLNs during dissection.
Materials needed for injection (8,9)
- ICG: 25 mg lyophilized dye in ampule;
- 10 cc of sterile water;
- 10 cc syringe;
- Two 5 cc syringe;
- 18 gauge hypodermic needle (for drawing up solution);
- 21 gauge spinal needle (for cervical injection).
Technique for injection
The dose of 0.5 mg/mL is created by a step by step dilution:
- Draw up 10 cc of sterile water in 10 cc syringe fitted with an 18 gauge hypodermic needle.
- Add 10 cc of sterile water to the ICG ampule and invert multiple times to obtain an adequate mixing.
- Draw up 4 cc of sterile water in each of the 5 cc syringe fitted with 18 gauge hypodermic needle.
- In each 5 cc syringe add 1 cc of the premixed ICG solution to obtain a total of 5 cc solution with a final concentration of 0.5 mg/mL.
- Discard 1 ml of ICG solution to obtain only 4 mL in each syringe; one syringe will be used for the first injection, the second one will be used for the potential re-injection in case of detection failure.
- Remove the 18 gauge hypodermic needle and replace it with the 21 gauge spinal needle.
- Insert the speculum to visualize the cervix and place the tenaculum on the anterior lip of the cervix.
- Insert the spinal needle to a 1 cm depth in the cervical stroma at 3 o’clock and inject 1 mL of solution; then retract the needle in the submucosa and inject another 1 mL; remove the needle and repeat the same steps on the opposite site at 9 o’clock.
ICG tracer is injected into the cervix after anesthesia induction and preparation of surgical field; after injection, pneumoperitoneum and port placement, side docking is performed to allow an easy access to the cervix in case of re-injection. The first surgical step is peritoneal evaluation and washing for cytological examination. Fluorescence imaging is used for transperitoneal visualization of ICG tracer in the lymphatic channels. In obese patients the transperitoneal evaluation is often negative therefore in these circumstances it is indicated to carefully develop the retroperitoneal spaces with care to not damage the lymphatic channels to avoid ICG spillage that can interfere with SLNs identification.
A successful mapping is defined by observing a channel leading from the cervix directly to at least one lymph node in at least one hemi-pelvis.
SLN is the first-in-chain nodes closest to the site of injection that has the highest likelihood of containing metastatic disease as it is the first to receive lymphatic drainage from the tumor.
SLN communicates with subsequent nodes along the same lymphatic channel pathways and that are defined upper echelon nodes.
We can recognize two common major lymphatic pathways of the uterus:
- Most common pathway (upper para-cervical pathway): lymphatics pass ventral to the uterine vessels and internal iliac artery before entering the nodes in the proximal obturator space at the bifurcation of the external and internal iliac vessels or on the medial surface of the external iliac vein (external iliac, inter-iliac and obturator nodes).
- Less common pathway (lower para-cervical pathway): cephalad, dorsal (or deep) to the ureter and enters the sacral promontory (pre-sacral and common iliac nodes) (9).
In Persson’s algorithm (10), it is mandatory to identify both pathways in each hemi-pelvis to declare the success of the procedure. Persson also divides the SLNs into three categories:
- ‘SLN type 1’ is the juxta-uterine fluorescent lymph node that receives an afferent lymphatic channel from each pathway.
- ‘SLN type 2’ is the non-fluorescent node that receives an afferent fluorescent lymphatic channel from each pathway. It should be considered that this type of SLN may represent a metastatic node as this do not always accumulate tracer.
- ‘SLN macro’ is any macroscopically suspect lymph node regardless of ICG mapping.
NCCN algorithm (9) states that all mapped nodes must be removed, however in our algorithm only the first-in-chain node for each hemi-pelvis is removed for each pathway when identified.
It is recommended switching frequently the fluorescence imaging into the real imaging to recognize correctly all anatomical landmarks for the dissection. According to the SLN level, is necessary to gently develop the retroperitoneal spaces (para-rectal space, para-vesical space and obturator fossa) without damaging, as much as possible, the lymphatic channels.
To remove the presacral SLNs the peritoneum is incised medial to the right common iliac artery to access the presacral avascular space; it is important to visualize the left common iliac vein that passes over L5 and to spare the hypogastric nerve.
To define the procedure as technically successful it is mandatory a bilateral detection at least of one of the two pathways (Video 1).
In case of unsuccessful uni- or bilateral SLN identification, an ipsi- or bilateral re-injection of ICG is performed with a minimum of 10 min’ observation time to allow the detection of the missing lymphatic pathways. Moreover all suspicious nodes must be removed regardless of mapping.
In our algorithm side specific pelvic lymphadenectomy (PL) in case of reinjection failure is mandatory only for intermediate or high risk tumor according with ESMO-ESGO guidelines classification (endometrioid G3, G1-G2 >50% myometrial invasion or non endometrioid histology). In these cases intraoperative macroscopic evaluation of myometrial invasion is performed by the pathologist. Frozen section of SLNs is not performed to not impair ultra-staging and therefore micro-metastases and isolated tumor cells (ITCs) detection and moreover in case of metastatic node we would not complete full lymphadenectomy.
However intraoperative pathological evaluation of SLN may be necessary to make sure of the presence of lymphatic tissue in the specimen, especially in case of obese patients.
In case of undetected bilateral pelvic (P) lymph-nodes is mandatory to explore para-aortic (PA) area to detect a real I level PA SLN, in order to identify a potential skip metastasis.
In NCCN algorithm (9) it was suggested the possibility to omit PA lymphadenectomy (PAL) in case of technical difficulties or in patients with low risk factors for PA nodal metastases.
In our algorithm no PA SLNs excision is performed if mapped PALN are II level LN, even for high risk tumor and no systematic PAL is recommended, unless suspicious, since adjuvant treatment is based on pelvic SLNs status and tumor risk factors (Figure 1).
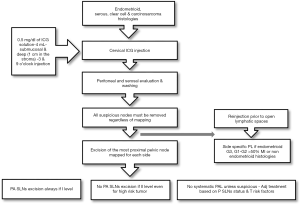
All SLNs are pathologically processed by ultra-staging using hematoxylin-eosin staining and immunohistochemistry.
SLNs are put in separate containers which are immediately labelled according to the different anatomical sites and handled separately in the pathology department. They are identified on palpation and dissected leaving peri-nodal adipose tissue for assessment of extra-nodal extension. Small lymph nodes up to 5 mm are embedded whole, those between 5 to 10 mm are bisected, the larger nodes are sliced in parallel slices at 3 mm interval, perpendicular to the long axis of the node. Paraffin blocks in dedicated (gray) colored cassettes are cut for ultra-staging at 3 levels at 50 microns, with two sections for each level, which are stained with hematoxylin & eosin and with immunohistochemistry using the anti-cytokeratin AE1/AE3 (Ventana Medical Systems, Inc., Tucson, AZ) for a total of 6 slides per block. Ultra-staging is performed as defined in the breast cancer literature by the American Joint Committee on Cancer. Macro-metastases are defined as metastases larger than 2.0 mm and micro-metastases as microscopic cell nests larger than 0.2 to 2 mm or less; ITCs are defined as metastatic carcinoma in the form of microscopic clusters and single cells measuring less than 0.2 mm.
Comments
SLN mapping algorithm has now replaced systematic PL and PAL in the management of apparent early EC. In a multicentre prospective trial ICG technique appeared feasible and reproducible, the accuracy reached the 99% of cases, with a false negative rate of 3% (8).
The recommendations (6) to ensure the optimal results of SLN mapping are:
- It is fundamental the expertise of surgeons, ensuring the adequate learning curve of all team.
- The most standardized technique consists in superficial and deep cervical injection of 2 mL of 0.5 mg/mL ICG solution at 3 and 9 o’clock.
- Systematic evaluation of the abdominal cavity is mandatory to exclude peritoneal metastases.
- SLN identification starts with the transperitoneal and then retroperitoneal visualization of lymphatic pathways that emerge from the parametria, followed by excision, for each hemi-pelvis, of the most proximal lymph nodes in at least one of two para-cervical pathways.
- Regardless of SLN mapping, any suspicious lymph nodes should be excised; in patient with high grade or non-endometrioid tumor the preoperative staging by imaging can guide surgical exploration.
- Performing routinely frozen section of SLNs is not justified due to its low sensitivity to detect low volume metastases in macroscopically normal appearing lymph nodes, more importantly to the potential alteration of ultra-staging pathology, and to the high costs.
- Performance of cervical re-injection and, in case of persistent mapping failure, performance of hemi-pelvic side-specific lymphadenectomy is mandatory to reduces false-negative staging.
- Performance of ultra-staging increases the detection of low-volume metastasis.
- Re-operation of positive SLN patients is not recommended considering the unsupported therapeutic intent of full lymphadenectomy, and the fact that adjuvant treatment is tailored on tumor risk factors (histology, grading, LVSI) and SLNs status.
Any potential microscopic residual disease in P an PA non SLNs is assumed to be cured by systemic adjuvant therapy.
There still are several challenges to face and solve, therefore a few randomized trials are under evaluation to study survival outcomes, to define the clinical significance and adequate treatment of low volume metastatic disease detected by ultra-staging and to evaluate the morbidity of SLNs excision compared with more comprehensive lymph nodes dissection.
Discussion
- Liliana Mereu: could the authors describe evidences for using ICG concentration of 0.5 mg/mL?
- Answer: the evidence for using ICG concentration of 0.5 mg/mL was based on pre-clinical animal lab and clinical data. (Rossi EC, Ivanova A, Boggess JF. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: a feasibility study. Gynecol Oncol 2012;124:78-82).
- Liliana Mereu: following your institution decision making to perform PL in case of unsuccessful SLN identification by intraoperative macroscopic evaluation, could you specify which factors are requested to the pathologist (myometrial infiltration, grading, volume) and which preoperative exams are performed to evaluate these factors?
- Answer: the patients undergo diagnostic hysteroscopy to confirm histology and grading, CT scan of the Chest and Abdomen to evaluate lymph node involvement or distant disease and MR of the pelvis to evaluate depth of myometrial invasion. In case of intraoperative macroscopic evaluation the pathologist is required to specify myometrial infiltration and volume of disease, grading is usually available preoperatively and it is not reliable when performed on frozen section.
- Liliana Mereu: could the authors underline advantages of robotic fluorescence SLN mapping?
- Answer: the SLN detection rates with ICG and the bilateral SLN detection rates, from the data of the literature, appear comparable or better than those of blue dye only or radiocolloid with a significantly shorter learning curve and a no need for preoperative Lymphoscintigraphy in case of radiocolloid. Fluorescent SLN localization with ICG is currently the preferred mapping approach at our institution and at many institutions worldwide.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Liliana Mereu) for the series “Robotic surgery for benign and malignant gynecological diseases” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-36/coif). The series “Robotic surgery for benign and malignant gynecological diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol 2008;109:11-8. [Crossref] [PubMed]
- ASTEC study group. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 2009;373:125-36. [Crossref] [PubMed]
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 2008;100:1707-16. [Crossref] [PubMed]
- Todo Y, Kato H, Kaneuchi M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet 2010;375:1165-72. [Crossref] [PubMed]
- Burke TW, Levenback C, Tornos C, et al. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study. Gynecol Oncol 1996;62:169-73. [Crossref] [PubMed]
- Holloway RW, Abu-Rustum NR, Backes FJ, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol 2017;146:405-15. [Crossref] [PubMed]
- Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol 2017;216:459-76.e10. [Crossref] [PubMed]
- Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 2017;18:384-92. [Crossref] [PubMed]
- Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:170-99. [Crossref] [PubMed]
- Persson J, Geppert B, Lönnerfors C, et al. Description of a reproducible anatomically based surgical algorithm for detection of pelvic sentinel lymph nodes in endometrial cancer. Gynecol Oncol 2017;147:120-5. [Crossref] [PubMed]
Cite this article as: Achilarre MT, Maramai M, Zanagnolo V. Robotic sentinel lymph node detection using indocyanine green. Gynecol Pelvic Med 2020;3:28.


