Diamond positioning and balloon dilation method: a new method for the establishment of inguinal working space in the resection of inguinal sentinel lymph nodes in patients with early stage vulvar cancer
Highlight box
Surgical highlights
• It’s a new method of marking the surgical area to achieve more accurate positioning.
• The balloon dilation method can dilate the surgical area slowly and bluntly, the procedure is fast and safe.
What is conventional and what is novel/modified?
• There is no standard method of making incision in laparoscopic inguinal sentinel lymph node resection, and it is difficult to establish the operating space quickly. we provide a method of incision positioning that can quickly and safely establish the operating space of the surgical area.
What is the implication, and what should change now?
• With the continuous progress of minimally invasive technology, gynecological oncologists are pursuing how to reduce surgical complications. Therefore, establishing a standardized operation process can shorten the learning curve and achieve better surgical effect. The approach we present in this study maybe a good attempt for establishing a standard practice.
Introduction
Vulvar cancer is a relatively rare malignant tumor of female genital organs, accounting for about 3–5% of female genital tumors, and it usually occurs in people over 60 years old (1). Lymphadenectomy is an important part of the surgical procedure for vulvar cancer. The traditional open inguinofemoral lymphadenectomy has a large surgical area, a lot of intraoperative bleeding, high probability of infection and necrosis after surgery. The incidence of postoperative complications is reported to be 70% or more (1,2), which seriously affect patient quality of life.
In recent publications, many authors have demonstrated the feasibility and safety of video endoscopic inguinal lymphadenectomy (VEIL), which has been found to produce a lower complication rate but equivalent oncologic outcome (3,4). Sentinel lymph node (SLN) biopsy is a less invasive technique than complete lymphadenectomy and significantly reduces the risk of lymphedema, wound infection, and dehiscence, and does not compromise survival rates or groin recurrence rates (5-7). Due to the low incidence of vulvar cancer, the short time of application of VEIL, and the fact that sentinel technique has not been widely carried out, there is currently no standard practice for the surgical incision location and the rapid exposure of the anatomical deconstruction of the surgical area. For beginners, due to lack of experience, it often takes a long time to establish the operating space in the groin femoral region, coupled with the possibility of bleeding and injury, which may affect the accurate identification of SLNs theoretically. We have currently developed a new method that enables accurate localization and rapid establishment of the operating space in the inguinal region. This method makes SLN excision more accurate and easier, shorting the operation time and facilitating rapid postoperative recovery.
In this case, a 54-year-old postmenopausal Tibetan woman sought medical treatment for vulva pain. Physical examination revealed a 2.2 cm diameter ulcer on the left vulva labia minora, and a biopsy indicated vulvar squamous cell carcinoma. magnetic resonance imaging (MRI) showed no enlarged lymph nodes in the groin and pelvis. According to the National Comprehensive Cancer Network guideline (8), a diagnosis of vulvar squamous cell carcinoma stage IB was considered. After communicating with the patient and signing the Informed consent, the radical resection of part of the vulva and biopsy of the left sentinel inguinal lymph node were performed. Indocyanine green was selected as a tracer in the inguinal SLN resection. The operation lasted for 70 minutes with an estimated blood loss of 20 mL. The surgery was performed in the operating room of a tertiary hospital. We present this article in accordance with the SUPER reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-7/rc).
Preoperative preparations and requirements
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Patient preparation
Vaginal scrubbing (1% povidone-iodine) was performed two days before surgery. Laxatives were taken orally and Vulvar skin preparation was performed 1 day before surgery.
Instrumental requirements
A 10-mm 30-degree standard-length optical camera for near infrared fluorescence imaging (TC 300, Karl STORZ, Germany), ultrasonic knife, 24-F foley catheter are required.
Surgical team
Three gynecologic oncologists, two experienced gynecologic surgical nurses and an anesthesiologist proficient in general anesthesia.
Surgical techniques
Step 1 Instrumental requirements: a 30° laparoscopy optic (TC 300, Karl STORZ, Germany), ultrasonic knife, 24-F foley catheter.
Patient position: the patient was placed in the supine position with abduction of the lower limbs. The groin was exposed. All procedures were performed under general anesthesia.
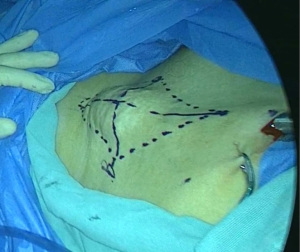
Step 2 Marking the surgical area: a connecting line was made between the lateral side of the pubic tubercle and the anterior superior iliac spine, about 10 cm in length; a vertical line through the midpoint of the line was made, about 12 cm in length. The four end points were successively connected to form a diamond-shaped surgical area. About 2 cm from the top vertex was marked as the point for placing the laparoscope. The medial incision was about 4 cm from the medial side of the diamond, and the lateral incision was about 4 cm from the lateral side of the diamond (Figure 1).
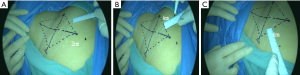
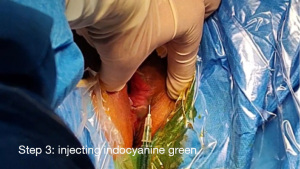
Step 3 Injecting indocyanine green. Indocyanine green was slowly injected subcutaneously at a distance of 0.5 cm from the vulvar mass, with a total of 3 injection sites, 0.5 mL each (Figure 2).
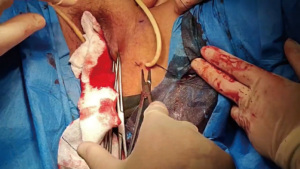
Step 4 Creating the working area. The tip of the 10 mm trocar was directed towards the lower apex of the diamond in the fat layer for puncture. The puncture was stopped about 3 cm beyond the midpoint, the puncture core was pulled out, and the 24-F foley catheter was inserted. Saline (60 mL) was slowly pushed into the water sac, making the surgical working area easily expanded (Figure 3).
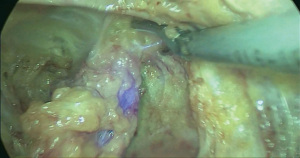
Step 5 SLN resection. Two 5-mm trocars were placed under laparoscopic monitoring and ultrasound knife was used to further enlarge the surgical area and expose the SLNs. The SLN was removed completely (Figure 4).
Step 6 Removing the SLN. The SLNs were bagged and removed from the vulva incision, and the drainage tube was indwelled in the perineum (Figure 5).
A new method for the establishment of inguinal working space in the resection of inguinal sentinel lymph nodes in patients with early stage vulvar cancer (Video 1).
Postoperative considerations and tasks
Prophylactic antibiotics were used for 48 hours after the operation. Skin temperature of the lower limbs as well as bilateral dorsalis pedis artery pulse be closely observed. External application of traditional Chinese medicine was given to the incision to promote wound healing. Intravenous analgesics were administered after operation. About 5 mL of fluid was drained daily, and the drain was removed 2 days after surgery. The patient was discharged successfully 7 days after the operation without complications.
Tips and pearls
- The puncture must be within the correct fascial layer to facilitate balloon dilation.
- If a bilateral groin lymphadenectomy is required, the laparoscopic hole can be placed closer to the lower abdominal midline, which allows for bilateral laparoscopic surgery and reduces the number of perforations;
- If sentinel-node mapping fails, ipsilateral lymphadenectomy should be performed instead.
Discussion
It’s a new method of marking the surgical area to achieve more accurate positioning. It could form a rhomboid operation area, which can not only meet the requirements of surgical operation, but also reduce the subcutaneous layer separation area than other method reported before, reduce the injury, and shorten the operation time. The balloon dilation method used in this study can slowly and bluntly dilate the surgical area, the procedure is fast and safe. At the same time, it can reduce the damage of blood vessels and lymphatics, improve the accuracy of SLN resection. In addition, the method of placing the drainage tube in the vulvar incision can improve the drainage efficiency and facilitate the abdominal wound healing after operation. SLN biopsy is suitable for patients with a single lesion less than 4 cm in diameter and no positive inguinal lymph node in clinical and/or imaging examinations, our method of locating and expanding the surgical area is suitable for all cases requiring inguinal lymphadenectomy.
Although this is a simple, safe and effective method, but it also has some disadvantages. If there is a scar in the surgical area, it is difficult to dilate the balloon, and separation by finger or ultrasonic knife is required. We use urinary balloon for dilation, which is convenient and cheap, easy to use in every hospital. However, when more than 50 mL of normal saline is injected, the urinary duct balloon ruptured sometimes. It would be better if we could have a special balloon with better elasticity. However, this procedure requires a gynecologic oncologist skilled in inguinal lymphadenectomy, when SLN dissection is required, the surgeon needs to be skilled in laparoscopic SLN imaging technique. Proficiency in our positioning and expansion technique requires only a few procedures.
There have been two types of incision approaches for VEIL for vulvar cancer, including VEIL using the hypogastric subcutaneous approach (VEIL-H), VEIL with the limb subcutaneous surgical approach (VEIL-L) (9). Both of these methods are minimally invasive, but still have some shortcomings. In VEIL-H, one of the incision is often located in the umbilicus. There is a long distance from the umbilicus to the groin, so a long section of subcutaneous tissue needs to be separated, causing unnecessary damage. VEIL-L can only be used in one groin area. When bilateral groin surgery is required, new incisions need to be made. There is also report of single-port laparoscopic surgery (10), but its application is limited by confined working space and requires the surgeon to be skilled in single-port laparoscopic technique. By contrast, the positioning method we used seems more accurate, less invasive and Easier. If a bilateral groin lymphadenectomy is required, the laparoscopic hole can be placed closer to the lower abdominal midline, which allows for bilateral laparoscopic surgery and reduces the number of perforations.
With the continuous progress of minimally invasive technology, gynecological oncologists are pursuing how to reduce surgical complications while ensuring the efficacy and safety of surgery. Therefore, establishing a standardized operation process after continuous exploration can shorten the learning period and achieve the best surgical effect. The approach we presented in our institution in this study may increase the possibility of establishing a standard practice. And more attempts need to be made.
Conclusions
Our new method of locating and expanding the surgical area in inguinal lymph node dissection can not only meet the needs of surgical operation, but also reduce bleeding and injury, which is conducive to rapid postoperative recovery and reduce postoperative complications. So far, we have carried out more than 10 operations with this method, with good results. The details of these cases will be reported in a subsequent article. However, more data should be collected to confirm its Long-term safety and effectiveness.
Acknowledgments
The video was awarded the second prize in the Third International Elite Gynecologic Surgery Competition (2022 Masters of Gynecologic Surgery).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Award-Winning Videos from the Third International Elite Gynecologic Surgery Competition (2022 Masters of Gynecologic Surgery)”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-7/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-7/coif). The series “Award-Winning Videos from the Third International Elite Gynecologic Surgery Competition (2022 Masters of Gynecologic Surgery)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weinberg D, Gomez-Martinez RA. Vulvar Cancer. Obstet Gynecol Clin North Am 2019;46:125-35. [Crossref] [PubMed]
- Vrielink OM, Faut M, Deckers EA, et al. Evaluation of the videoscopic inguinal lymphadenectomy in melanoma patients. Eur J Surg Oncol 2019;45:1712-6. [Crossref] [PubMed]
- Le A, Xiong J, Wang Z, et al. Endoscopy-assisted inguinal lymphadenectomy in vulvar cancer. Arch Gynecol Obstet 2018;297:1277-83. [Crossref] [PubMed]
- Postlewait LM, Farley CR, Diller ML, et al. A Minimally Invasive Approach for Inguinal Lymphadenectomy in Melanoma and Genitourinary Malignancy: Long-Term Outcomes in an Attempted Randomized Control Trial. Ann Surg Oncol 2017;24:3237-44. [Crossref] [PubMed]
- Te Grootenhuis NC, van der Zee AG, van Doorn HC, et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol 2016;140:8-14. [Crossref] [PubMed]
- Gitas G, Proppe L, Baum S, et al. A risk factor analysis of complications after surgery for vulvar cancer. Arch Gynecol Obstet 2021;304:511-9. [Crossref] [PubMed]
- Cibula D, Oonk MH, Abu-Rustum NR. Sentinel lymph node biopsy in the management of gynecologic cancer. Curr Opin Obstet Gynecol 2015;27:66-72. [Crossref] [PubMed]
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:92-120. [Crossref] [PubMed]
- Le A, Xiong J, Wang Z, et al. Endoscopy-assisted inguinal lymphadenectomy in vulvar cancer. Arch Gynecol Obstet 2018;297:1277-83. [Crossref] [PubMed]
- Xu J, Duan K, Guan X, et al. Laparoendoscopic single-site inguinal lymphadenectomy in gynecology: preliminary experience at a single institution. Arch Gynecol Obstet 2020;302:497-503. [Crossref] [PubMed]
Cite this article as: Wei Y, Chen Y, Lin Y. Diamond positioning and balloon dilation method: a new method for the establishment of inguinal working space in the resection of inguinal sentinel lymph nodes in patients with early stage vulvar cancer. Gynecol Pelvic Med 2023;6:16.