
The dedicator of cytokinesis 6 (DOCK6) is a novel indicator for prognosis in cervical cancer: a retrospective cross-sectional study
Highlight box
Key findings
• Patients with high dedicator of cytokinesis 6 (DOCK6) expression had a poorer overall survival (OS) in compared with the low DOCK6 expression group.
What is known and what is new?
• DOCK6 is associated with cell proliferation and metastasis in certain types of cancer and could serve as a biomarker for cancer prognosis. But its expression and pathophysiological effect in cervical cancer (CC) stays unknown. In this study, we found the expression of DOCK6 was significantly associated with the lymph node metastasis, the depth of stromal invasion, the positive vaginal cuff and lymphovascular space invasion. Besides, patients with high DOCK6 expression had a poorer OS in compared with the low DOCK6 expression group.
What is the implication, and what should change now?
• Combined, DOCK6 was probably involved in the development and progression of CC, for which DOCK6 could be a potential target for immunotherapy for CC in the future.
Introduction
Cervical cancer (CC) is the fourth leading cancer affected women globally. In the age between 20 to 39, CC is the second most common cause of cancer death in 2019 (1). According to the report from World Health Organization in 2022, an estimated 604,000 new cases and 342,000 deaths happened in 2020 (1). The prognosis of CC varies based on the clinical stage, status of the lymph nodes, tumor volume, depth of tumor invasion into the cervical stroma, and lymph-vascular space invasion (2). The 5-year survival of patients with stage IB node-negative disease is up to 87%, while it is approximately 70% for those with locally advanced CCs (3). For early stage, either surgical treatment or concurrent chemoradiotherapy is appropriate, but for the advanced stage, concurrent chemoradiotherapy is superior (4). Immunotherapy is another therapeutic approach to target a specific signaling pathway in cells. Progress in immunotherapy research and the expression of biomarkers in CC offers new hope for patients with advanced stage and recurrent or metastatic disease (5-7). Currently, the Pembrolizumab, an immune checkpoint programmed cell death protein 1 (PD-1) inhibitor, is the only drug approved by the US Food and Drug Administration to apply in the advanced stage with progressive disease (8,9). Some research also reveals the use of biomarkers in CC is promising (10,11).
The dedicator of cytokinesis (DOCK) is a conserved family which work as atypical guanine nucleotide exchange factors (GEFs) to participate in the cellular signaling pathway (12). The family consists of 11 gene products (DOCK 1–11) that are structurally divided into four classes as DOCK-A, -B, -C, and -D (13). DOCK6 is a member of DOCK-C subfamily, and it displays GEF activities for both Rac1 and Cdc42 through its Dock Homology Region-2 (DHR-2) domain both in vitro and in vivo (14). Rac1 and Cdc4 are well-known members of the Rho GTPase family, that play an important role in cell activities including tumorigenesis, angiogenesis, invasion, and metastasis (15,16). In gastric cancer, DOCK6 participates in cell migration, proliferation, sphere formation and tumorigenicity, and promotes chemo- and radioresistance through Wnt/β-catenin signaling pathway (13,16). Down-regulation of DOCK6 promotes gastric cancer proliferation and metastasis trough activation of Rac1 and Cdc42 both in vivo and in vitro (17). According to the research above, DOCK6 could serve as a potential therapeutic target for tumor therapy. However, the expression pattern of DOCK6 in CC has not been demonstrated yet and its molecular biological functions in the tumor remains unknown.
In the present study, immunohistochemical analysis was performed to assess the expression of DOCK6 in CC and its prognostic value in different stages of CC was evaluated. We present this article in accordance with the STROBE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-13/rc).
Methods
Patients and tissue sample
In this retrospective cross-sectional study, archived formalin-fixed paraffin-embedded specimens from 94 CC patients were included between June 2006 and June 2012, during which period patients’ medical records, cervical tissues and follow-up records were fully collected and well preserved. Only CC patients without chemotherapy or radiotherapy before surgical treatment were enrolled. Patients with neoadjuvant chemotherapy or combined with tumors in other organs were excluded. The telephone follow-ups were completed with two individual staff. Three control cervical tissues were from females who underwent hysterectomy because of uterine myoma. Those tissue slides including malignant and normal cervical tissues were obtained from the Department of Pathology West China Second University Hospital, Sichuan University (Chengdu, China). The characteristics of patients, including age, histological type and disease stage are summarized in Table 1.
Table 1
| Characteristics | Total, n (%) | DOCK6-positive tumor cells | ||
|---|---|---|---|---|
| Low, n (%) | High, n (%) | P value | ||
| Total number | 94 | 55 (58.51) | 39 (41.49) | |
| Age (years) | 0.375 | |||
| ≤65 | 91 (96.81) | 52 (55.32) | 39 (41.49) | |
| >65 | 3 (3.19) | 3 (3.19) | 0 (0.00) | |
| Stage | 0.34 | |||
| ≤ IIB | 82 (87.23) | 50 (53.19) | 32 (34.04) | |
| > IIB | 12 (12.77) | 5 (5.32) | 7 (7.45) | |
| Grade | 0.11 | |||
| Low (G1 + G2) | 84 (89.36) | 52 (55.32) | 32 (34.04) | |
| High (G3 + G4) | 10 (10.64) | 3 (3.19) | 7 (7.45) | |
| Lymph node metastasis | 0.032* | |||
| Negative | 77 (81.91) | 49 (52.13) | 28 (29.79) | |
| Positive | 17 (18.09) | 6 (6.38) | 11 (11.70) | |
| Histologic type | 0.207 | |||
| Squamous carcinoma | 83 (88.03) | 51 (54.26) | 32 (34.04) | |
| Non-squamous carcinoma | 11 (11.70) | 4 (4.26) | 7 (7.45) | |
| Stromal invasion (depth) | 0.014* | |||
| ≤1/2 | 43 (45.74) | 31 (32.98) | 12 (12.77) | |
| >1/2 | 51 (54.26) | 24 (25.53) | 27 (28.72) | |
| Vaginal cuff involvement | 0.032* | |||
| Negative | 75 (79.79) | 48 (51.06) | 27 (28.72) | |
| Positive | 19 (20.21) | 7 (7.45) | 12 (12.77) | |
| Lymphovascular space invasion | 0.009* | |||
| Negative | 48 (51.06) | 34 (36.17) | 14 (14.89) | |
| Positive | 46 (48.94) | 21 (22.34) | 25 (26.60) | |
| Parametrium involvement | 0.184 | |||
| Negative | 89 (94.68) | 54 (57.45) | 35 (37.23) | |
| Positive | 5 (5.32) | 1 (1.06) | 4 (4.26) | |
| Tumor-specific survival | 0.046* | |||
| Total/events/censored | 94/10/84 | 55/3/52 | 39/7/32 | |
| Median survival | 75.83±1.69 | 79.62±0.79 | 64.72±3.35 | |
| 95% confidence interval | 72.51–79.15 | 78.08–81.17 | 58.14–71.29 | |
*, P<0.05. Events, cancer-associated mortality; Censored, patients were alive at the date of the last visit or at the time of mortality due to non-cervical cancer-associated causes. DOCK6, dedicator of cytokinesis 6.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of West China Second Hospital of Sichuan University (2023 Medical Scientific Research for ethical approval No. 316) and written informed consent was obtained from all patients.
Immunohistochemistry (IHC) analysis of DOCK6 expression
DOCK6 expression in different CC tissues was analyzed by immunohistochemical method. The paraffin-embedded tissues were sectioned (thickness, 3–4 µm) and mounted on poly-l-lysine-coated slides. Firstly, samples were deparaffinized with 99% (v/v) xylene and sequentially rehydrated in a graded ethanol series (100%, 95%, 80% and 50%). Antigen retrieval was performed using 10 mmol/L boiling (~95 ℃) sodium citrate buffer at pH 6.0 for 15 min. Samples were immersed in 3% hydrogen peroxide for 30 min at room temperature to block the endogenous peroxidase activity, followed by incubation in 5% bovine serum albumin (BSA) (cat. no. 9048-46-8; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min to reduce non-specific binding. Slides were then sequentially incubated with the primary antibodies, anti-human DOCK6 polyclonal rabbit antibody (cat. no. 25087-1-AP; Proteintech, Wuhan, China; 1:250 diluted in 5% BSA) at 4 ℃ overnight and then incubated with a secondary horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (cat. no. A0208; Beyotime, Shanghai, China) at room temperature for 1 h. After that, slides were then incubated with 1% (w/v) 3,3’-diaminobenzidine solution to develop color. Finally, slides were counter-stained with 0.5% (w/v) hematoxylin and covered with neutral balsam. Images were acquired using a Carl Zeiss Axiovert 200M microscope and AxioVision Rel. 4.7 software.
Evaluation of DOCK6 protein expression
The evaluation of the protein expression in CC tissues was analyzed with a semi-quantitative method. The method was based on the number of positive tumor cells and the staining intensity (regardless of the positive subcellular location) (18). Briefly, the DOCK6 staining intensity was classified as follows: 0, negative staining; 1, weak staining (light yellow); 2, moderate staining (yellow-brown); and 3, strong staining (brown). In the same tumor tissue with different staining intensities, only the highest intensity was adopted. The percentage of DOCK6-positive cells was also scored as follows: 0, no stained cells; 1, 1–30% positive cells; 2, 31–60% positive cells; 3, 61–90% positive cells; 4, 91–100% positive cells. The final immunoreactivity score (IS) of each sample was determined by adding the scores for the staining intensity and the percentage of DOCK6-positive cells. Scores of 0–3 were defined as ‘negative expression’ (−), scores of 4–5 as ‘weakly positive expression’ (+), and scores of 6–7 as ’strongly positive expression’ (++). In addition, overall scores were dichotomized into two groups: low expression (IS <5); and high expression (IS ≥5) in CC samples.
Statistical analysis
In the present study, patients were followed until the end of the follow-up period (June 2012) or mortality. The overall survival (OS) was reckoned from the date of the initial diagnosis to the date of the last follow-up or mortality. Patients were censored at the date of the last visit or mortality due to non-CC-associated causes. The correlation between the clinicopathological characteristics of patients and the expression of DOCK6 in CC was analyzed using Chi-square test or Fisher’s exact test with SPSS v22 software (IBM Corp., Armonk, NY, USA). OS was evaluated via Kaplan-Meier 5-year survival curves using log-rank tests. Univariate and multivariate Cox proportional hazard models were applied to measure the associations between DOCK6 expression and clinical characteristics with OS. P<0.05 (two-tailed) was considered to indicate a statistically significant difference.
Results
Patients’ characteristics
A total of 94 patients with CC were brought into this study. The median follow-up period was 56 months (ranging from 11 to 74 months). At the end of the 6-year study period, 86 cases of survival were censored, while the other eight events were CC-associated mortalities. The eight patients had relapse and three of them had experienced metastasis before death. General characteristics of patients were displayed in Table 1. Generally, women who are 65 years old or older could stop screening for negative screening results for her past 10 years, thus we divided our patients into two age groups (≤65 years, n=91, 96.81% and >65 years, n=3, 3.19%). The median age was 41.2 years with range from 31 to 67 years, and 96.81% of them were equal to or lower than 65 years. The cervical stage was divided according to International Federation of Gynecology and Obstetrics (FIGO) staging [1994] (19). According to the National Comprehensive Cancer Network (NCCN) guideline, definitive chemoradiation is recommended for most advanced stage (> stage IIB). Thus, patients here were divided into early-stage group (≤ IIB) (n=82, 87.23%) and advanced stage (> stage IIB) (n=12, 12.77%). Overall, 88.30% (n=83) of cases were cervical squamous cell carcinoma, and the rest 11.70% (n=11) were non-squamous carcinoma. The pathological grade was classified into two groups, with 10.64% (n=10) poorly differentiated as Grade 3/Grade 4, 89.36% (n=84) were relatively well differentiated as Grade 1/Grade 2.
Expression of DOCK6 in cervical lesions and the related clinical relationships detected by IHC
The expression of DOCK6 mainly located in the cytoplasm (Figure 1), which was in accordance with its cellular function. The final IS of each sample was based on a combination of the percentage of DOCK6-positive cells and the staining intensity. High expression of DOCK6 (IS ≥5) was detected in 39 samples (41.49%), and the rest 55 samples (58.51%) were deemed as low expression group. The association between patients’ clinicopathological characteristics and DOCK6 expression (low vs. high expression) was summarized in Table 1. Generally, DOCK6 expression was significantly associated with lymph node metastasis (P=0.032), the depth of stromal invasion (P=0.014), the positive vaginal cuff (P=0.032), lymphovascular space invasion (P=0.009). Specifically, patients with lymph node metastasis (11.70% vs. 6.38%), involvement of vaginal cuff (12.77% vs. 7.45%) and lymphovascular space invasion (26.60% vs. 22.34%) were more common to have high DOCK6 expression than those without. Moreover, patients with stromal invasion over 1/2 were prone to have high DOCK6 expression than those with stromal invasion less than 1/2 (28.72% vs. 25.53%). However, the DOCK6 expression was not significantly associated with the patients’ age, cancer stage, the tumor cell differentiation, the histologic type and the parametrium involvement.
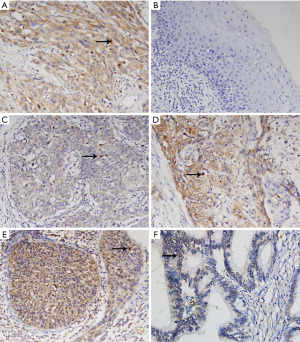
Survival analysis and prognostic significance of DOCK6 in CC
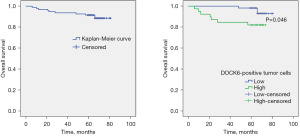
To investigate the potential relationship between DOCK6 and the prognosis of CC, Kaplan-Meier curves was constructed for OS analysis. The total median survival time was 75.83±1.69 months and the overall 5-year survival rate was as high as 90.42%. The median survival time in patients with high DOCK6 expression (n=39) was significantly shorter than that in the low DOCK6 expression group (n=55) (64.72±3.35 vs. 79.62±0.79 months) (P=0.046). As shown in Figure 2, the 5-year OS rate of the high DOCK6 expression group was 82.05%, significantly lower than that of the low DOCK6 expression group (96.36%).
Moreover, univariate Cox regression model was performed to explore the associations between the clinicopathological characteristics and OS in CC patients. As displayed in Table 2, results suggested the cancer stage [hazard ratio (HR) =9.13, P=0.001], lymph node status (HR =6.89, P=0.030), and the DOCK6 expression (HR =3.62, P=0.046) were correlated a shorter OS and poor prognosis. When taken those four factors together into consideration, the multivariate Cox regression model results revealed only these three factors cancer stage (HR =10.67, P=0.008), lymph node status (HR =9.01, P=0.002), and the DOCK6 expression (HR =7.68, P=0.030) were independent factors to predict the prognosis of CC. Specifically, the advanced cancer stage (> IIB), the positive lymph node metastasis and high expression of DOCK6 in tumor tissue all indicated a poor survival for CC patients.
Table 2
| Characteristics | N | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Age (years) | 0.31 (0.04–2.42) | 0.263 | 0.05 (0.01–1.56) | 0.873 | ||
| ≤65 | 91 | |||||
| >65 | 3 | |||||
| Stage | 9.13 (2.63–31.68) | 0.001* | 10.67 (1.88–60.61) | 0.008* | ||
| ≤ IIB | 82 | |||||
| > IIB | 12 | |||||
| Grade | 1.12 (0.14–8.87) | 0.912 | 2.12 (0.08–53.97) | 0.650 | ||
| Low (G1 + G2) | 84 | |||||
| High (G3 + G4) | 10 | |||||
| Lymph node metastasis | 6.89 (1.94–24.45) | 0.030* | 9.01 (2.20–36.95) | 0.002* | ||
| Negative | 77 | |||||
| Positive | 17 | |||||
| Histologic type | 1.98 (0.42–9.36) | 0.388 | 1.01 (0.11–9.01) | 0.101 | ||
| Squamous carcinoma | 83 | |||||
| Non-squamous carcinoma | 11 | |||||
| Stromal invasion (depth) | 1.27 (0.36–4.50) | 0.172 | 0.86 (0.18–4.14) | 0.856 | ||
| ≤1/2 | 43 | |||||
| >1/2 | 51 | |||||
| Vaginal cuff involvement | 2.71 (0.76–9.60) | 0.123 | 0.44 (0.06–3.11) | 0.415 | ||
| Negative | 75 | |||||
| Positive | 19 | |||||
| Lymphovascular space invasion | 2.55 (0.66–9.86) | 0.175 | 2.48 (0.44–14.00) | 0.304 | ||
| Negative | 48 | |||||
| Positive | 46 | |||||
| Parametrium involvement | 2.00 (0.25–15.84) | 0.510 | 1.01 (0.11–9.07) | 0.964 | ||
| Negative | 89 | |||||
| Positive | 5 | |||||
| DOCK6 expression | 3.62 (0.94–14.00) | 0.046* | 7.68 (1.22–48.37) | 0.030* | ||
| Low | 55 | |||||
| High | 39 | |||||
*, P<0.05. N, number; HR, hazard ratio; CI, confidence interval; DOCK6, dedicator of cytokinesis 6.
Discussion
CC is one of a few cancers which has a clear cause responsible for its development. More than 99.7% of invasive cervical squamous cell is related to the infection of human papillomavirus (HPV) in genital tract (20). Nowadays, CC is considered as a preventable cancer due to the wide use of HPV vaccination. The progress of CC is a long-term process starting from HPV infection, followed by involvement of several cell signaling pathway (21,22). Several distinct signaling pathways that have been implicated in the progress of HPV-induced cancer, which may provide the potential targets for immunotherapy. The Wnt/β-catenin pathway may be critical for the initiation of CC. The E6 oncoprotein of HPV16 activates the Wnt/β-catenin pathway and result in the accumulation of β-catenin, which induces transcription of a wide variety of genes to promote cell proliferation and differentiation (23). Another important pathway is PI3K/Akt pathway. Once being activated by E6/E7, PI3K/Akt pathway could lead to carcinogenesis by upregulation of EGFR and subsequently activation of MAPK/ERK pathways (24).
With the extensive disclosure of the interaction between tumor microenvironment and immune cells, immunotherapy soon became a promising therapeutic approach which plays roles through immunomodulatory signaling pathways (25). Immune check-point inhibitors are the most successful immunotherapy that magnify the immune responses to heterogeneous tissues (26). At present, the most famous check-points are PD-1 and its ligand (PD-L) and the cytotoxic T lymphocyte associate antigen 4 (CTLA-4), which impair the activation, proliferation and anti-tumor function of T cells (27,28). Based on that, drugs like ipilimumab targeting CTLA-4, pembrolizumab and nivolumab targeting PD-1 are developed and applied in certain type of cancers (29). According to the preliminary results, those three drugs showed attractive antitumor activity in CC alone or combined with chemotherapy/radiotherapy (6).
DOCK6 is a member of DOCK family, which are evolutionary conserved exchange factors for the Rho GTPases Rac1 and Cdc42. In human body, DOCK6 is mainly expressed in fat, followed by lung and thyroid. DOCK6 has two domains and regulates neurite outgrowth through the second domain, which function as the GEF for Cdc42 and Rac1 small GTPases (14). Wang et al. firstly revealed the DOCK6 expression is an independent biomarker for gastric cancer prognosis through Wnt/β-catenin signaling pathway (30). Furthermore, overexpression of DOCK6 in gastric cancer cell leaded to the lower chemo- and radio-sensitivities and induction of stemness phenotypes (31). Except for gastric cancer, the role of DOCK6 as a prognostic indicator was also demonstrated in another cancer oral squamous cell cancer, thus enhancing its potential therapeutic target for cancer treatment (32). Being activated by DOCK6, Rac1 and Cdc42 then regulate β-catenin to modulate tumor progression through Wnt signaling, which pathway could be suppressed by microRNA miR-148b-3p (17). As illustrated previously, Wnt/β-catenin signaling pathway and PI3K/Akt pathway play role in development and progression of CC, based on which point we presumed the DOCK6 is presumably associated with CC. Similar to Chi’s finding (31), in our research, we found high DOCK6 expression was correlated with the lymph node metastasis (P=0.032), the depth of cervical stromal invasion(P=0.004), parametrial infiltration(P=0.032), the depth of stromal invasion (P=0.014), the vaginal cuff involvement (P=0.032) and lymphovascular space invasion (P=0.009). Those results suggested DOCK6 may be associated with the progress of tumor cells, which means target on DOCK6 could be a good choice against disease progression. Moreover, according to the univariate and multivariate Cox regression analyses, DOCK6 was an independent factor to predict the prognosis of CC, and the high DOCK6 expression in tumor tissue possibly indicates a poor prognosis.
Those combined results indicate the DOCK6 may involve in the progression of CC, providing the possibility that DOCK6 works as a potential target for CC therapy. Thus, the measurement of DOCK6 expression in the patients’ biopsy tissue or surgical specimens may be effective to evaluate the stage of CC and predict the prognosis. More importantly, the measurement of DOCK6 expression is an appropriate way to identify the specific patients who would benefit from the novel DOCK6-associated immunotherapy and contribute to the development of personalized treatment programs. However, this was small retrospective cross-sectional study in one center, which added bias in the conclusion. In the future, we are aiming to investigate the pathway of DOCK6 playing in CC in cell research and try to get more clinicopathological data from multiple centers.
Conclusions
DOCK6 was expressed in CC tissue and its expression was highly correlated with the tumor-specific survival of CC. High expression of DOCK6 was more commonly found in advanced stage patients, and it was identified as an independent prognostic factor for the prediction of poor survival in CC. Thus, DOCK6 was probably involved in the development and progression of CC, for which DOCK6 could be a potential target for therapy for CC in the future. Moreover, the measurement of DOCK6 in CC tissue could help predict the prognosis of patients and provide evidence for personalized immunotherapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-13/rc
Data Sharing Statement: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-13/dss
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of West China Second Hospital of Sichuan University (2023 Medical Scientific Research for ethical approval No. 316) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606-13. [Crossref] [PubMed]
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169-82. [Crossref] [PubMed]
- Abu-Rustum NR, Yashar CM, Bean S, et al. NCCN Guidelines Insights: Cervical Cancer, Version 1.2020. J Natl Compr Canc Netw 2020;18:660-6. [Crossref] [PubMed]
- Orbegoso C, Murali K, Banerjee S. The current status of immunotherapy for cervical cancer. Rep Pract Oncol Radiother 2018;23:580-8. [Crossref] [PubMed]
- Ferrall L, Lin KY, Roden RBS, et al. Cervical Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res 2021;27:4953-73. [Crossref] [PubMed]
- Otter SJ, Chatterjee J, Stewart AJ, et al. The Role of Biomarkers for the Prediction of Response to Checkpoint Immunotherapy and the Rationale for the Use of Checkpoint Immunotherapy in Cervical Cancer. Clin Oncol (R Coll Radiol) 2019;31:834-43. [Crossref] [PubMed]
- FDA approves pembrolizumab combination for the first-line treatment of cervical cancer. FDA [Internet]. 2022 Jan 31 [cited 2022 Sep 18]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-combination-first-line-treatment-cervical-cancer
- Chung HC, Schellens JHM, Delord JP, et al. Pembrolizumab treatment of advanced cervical cancer: Updated results from the phase 2 KEYNOTE-158 study. J Clin Oncol 2018;36:5522. [Crossref]
- Cao Y, Zhou X, Huang X, et al. Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS One 2013;8:e53834. [Crossref] [PubMed]
- Kosmaczewska A, Bocko D, Ciszak L, et al. Dysregulated expression of both the costimulatory CD28 and inhibitory CTLA-4 molecules in PB T cells of advanced cervical cancer patients suggests systemic immunosuppression related to disease progression. Pathol Oncol Res 2012;18:479-89. [Crossref] [PubMed]
- Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci 2002;115:4901-13. [Crossref] [PubMed]
- Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol 2014;93:466-77. [Crossref] [PubMed]
- Miyamoto Y, Yamauchi J, Sanbe A, et al. Dock6, a Dock-C subfamily guanine nucleotide exchanger, has the dual specificity for Rac1 and Cdc42 and regulates neurite outgrowth. Exp Cell Res 2007;313:791-804. [Crossref] [PubMed]
- Bid HK, Roberts RD, Manchanda PK, et al. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther 2013;12:1925-34. [Crossref] [PubMed]
- Xiao XH, Lv LC, Duan J, et al. Regulating Cdc42 and Its Signaling Pathways in Cancer: Small Molecules and MicroRNA as New Treatment Candidates. Molecules 2018;23:787. [Crossref] [PubMed]
- Li X, Jiang M, Chen D, et al. miR-148b-3p inhibits gastric cancer metastasis by inhibiting the Dock6/Rac1/Cdc42 axis. J Exp Clin Cancer Res 2018;37:71. [Crossref] [PubMed]
- Liao H, Zhu H, Liu S, et al. Expression of V-domain immunoglobulin suppressor of T cell activation is associated with the advanced stage and presence of lymph node metastasis in ovarian cancer. Oncol Lett 2018;16:3465-72. [Crossref] [PubMed]
- Odicino F, Pecorelli S, Zigliani L, et al. History of the FIGO cancer staging system. Int J Gynaecol Obstet 2008;101:205-10. [Crossref] [PubMed]
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12-9. [Crossref] [PubMed]
- Gupta S, Kumar P, Das BC. HPV: Molecular pathways and targets. Curr Probl Cancer 2018;42:161-74. [Crossref] [PubMed]
- Gutiérrez-Hoya A, Soto-Cruz I. Role of the JAK/STAT Pathway in Cervical Cancer: Its Relationship with HPV E6/E7 Oncoproteins. Cells 2020;9:2297. [Crossref] [PubMed]
- Lichtig H, Gilboa DA, Jackman A, et al. HPV16 E6 augments Wnt signaling in an E6AP-dependent manner. Virology 2010;396:47-58. [Crossref] [PubMed]
- Rodon J, Dienstmann R, Serra V, et al. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 2013;10:143-53. [Crossref] [PubMed]
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015;125:3335-7. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987;328:267-70. [Crossref] [PubMed]
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-95. [Crossref] [PubMed]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19:813-24. [Crossref] [PubMed]
- Wang CS, Tsai CY, Lee KF, et al. Association of DOCK6 with cancer stem cell development and as an independent prognostic factor of gastric cancer. J Clin Oncol 2017;35:68. [Crossref]
- Chi HC, Tsai CY, Wang CS, et al. DOCK6 promotes chemo- and radioresistance of gastric cancer by modulating WNT/β-catenin signaling and cancer stem cell traits. Oncogene 2020;39:5933-49. [Crossref] [PubMed]
- Zhang ZY, Sun YY, Wang HC, et al. Overexpression of DOCK6 in oral squamous cell cancer promotes cellular migration and invasion and is associated with poor prognosis. Arch Oral Biol 2022;133:105297. [Crossref] [PubMed]
Cite this article as: Liao H, Wang Q, Zou J, Tan X. The dedicator of cytokinesis 6 (DOCK6) is a novel indicator for prognosis in cervical cancer: a retrospective cross-sectional study. Gynecol Pelvic Med 2023;6:19.


