
Numb suppression promoted the pathogenesis of SiHa cells by stimulating Notch and Hedgehog signaling pathways
Highlight box
Key findings
• Numb inhibition promotes malignant transformation of cervical squamous carcinoma through stimulation of Notch and Hedgehog-dependent pathways.
What is known and what is new?
• The current research on Numb is mainly focused on the development of the nervous system.
• We found that ectopic expression of Numb was an important factor in the progression of cervical cancer and that low Numb expression levels can be used as an indicator of the prognosis of squamous cervical cancer.
What is the implication, and what should change now?
• Numb reduction is an important contributor to cervical cancer progression, rationalizing low expression level of Numb may be a predictor to the prognosis of cervical squamous carcinomas.
Introduction
Cervical cancer is the prevalent life-threatening disease diagnosed in the female population today. Cervical cancer still the commonest cause of cancer deaths among women in developing counties with large geographic variations in cancer incidence and mortality rates (1-5). Detection of cervical cancer at an early stage is associated with excellent survival. However, patients with advanced cervical cancer had a higher mortality, especially in developing countries. The ratio between incidence and mortality from cervical cancer remains high due to lack of access to appropriate anti-cancer therapies (6-8).
Previous study indicated that human papillomavirus infection has been the major causes in most cervical cancer cases and genetic alterations play the key role for tumor genesis and progression (9-11). Recent advances in the biology of cervical cancer revealed genetic changes is common in cervical carcinogenesis and metastasis (12-14). Genetic alteration due to point mutation and chromosome translocation has been extensively studied in cervical cancer (15-17). Recent research strategies keep insight into tumor suppressors. Meanwhile, significance has been grown to study the utility of these changes as biomarkers to determine disease progression as well as use them as therapeutic targets (18-21).
The human Numb gene is located at chromosome 14q24.3 which was known as the “cell fate determinant” and its encoded protein is the first signal protein which was found to regulating cell asymmetric division (22-24). The current research on Numb is mainly focused on the development of the nervous system (24-27). Numb is involved in the directed differentiation of neural precursor cells. It is asymmetrically distributed into two daughter cells during cell division (28-30). More recently, studies have shown that asymmetric division of cell fate determinants can affect cell proliferation and differentiation (31-34). Which implied the function of Numb may be related to tumorigenesis or progression.
Here, we investigated the involvement of Numb in development of cervical squamous carcinoma progression. In the study, we showed that repression of Numb in cervical squamous carcinoma cells lead to malignant progression in vitro and in vivo. Further mechanistic studies identified the Numb reduction promoted cervical squamous carcinoma malignant progression through Notch and Hedgehog pathways. Meanwhile, the clinical evidences we provided were in correspondence with our experiments of Numb function. In summary, our data provided mechanistic insight into Numb reduction-induced malignant transformation, enhance our understanding of the events in progression of cervical squamous carcinoma and establish Numb reduction as a viable predictor to the prognosis of cervical squamous carcinomas. We present this article in accordance with the ARRIVE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-30/rc).
Methods
Materials, cell lines and agents
HaCaT (ATCC®PCS-200-011TM) and SiHa (ATCC®HTB-35TM) cells were from the American Type Culture Collection (ATCC; Rockville, MD, USA) and these cells were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Caoyuan lvye, Huhht, China). YOYO-1, Hoechst 33342, were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA). Horseradish peroxidase (HRP)-labeled goat anti-rabbit, anti-mouse secondary antibodies or anti-Numb antibody were provided by Cell Signaling Technology (Notch1, 70109; Hes, 11988; Shh, 2207) (Danvers, MA, USA) and Gene Tex (Smo, GTX128298) (California, USA). All other chemicals were purchased from Sigma-Aldrich (Shanghai, China) and used without further purification.
Cervical tissue samples
Between January 2019 and October 2021, we collected 25 normal cervical epithelial tissue specimens, 60 CIN tissue specimens, 40 cervical invasive cancer tissue specimens and 15 cervical cancer lymph node tissue specimens. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of West China Second Hospital of Sichuan University (No. 2020126) and informed consent was obtained from all individual participants. Cervical cancer tissue chips CR806 and CR2085 were purchased from Xi’an Elena Biotechnology Co., Ltd. 163 cases of cervical squamous carcinoma and 6 cases of lymph node metastasis of cervical squamous carcinoma were involved in the analysis.
Preparation of SiHaNumb− cell line
First, a custom shRNA plasmid was designed to target human Numb gene (Open Biosystems). We screened one of the four shRNAs achieving 90% knockdown efficiency by RT-PCR. The sequence of Numb interference sites: CACATTTACTGAAATGTAA. Subsequently, the lentiviral transduction was performed as described (35). Briefly, 293 T cells were co-transfected with pMD2.G, pPAX2 (Addgene), and pGIPZ shRNA plasmids. For infections, cells were incubated with viral supernatants in the presence of 8 µg/mL polybrene. After 48 h, the infected cells were selected with puromycin (2 µg/mL) for SiHaNumb− clones for 3–4 days and confirmed by western blot before experiments.
Xenograft experiments in vivo
Female Balb/c nude mice (4–5 weeks) were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). The animals were used for anti-tumor tests in vivo, which were housed at a controlled temperature of 20–22 °C, relative humidity of 50–60% and 12 h light-dark cycles. Five-week-old female nude mice were obtained from Beijing HFK Bioscience Co., Ltd. (Beijing, China). All animal experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals, approved by the Ethics Committee of Sichuan University (Chengdu, China). SiHa and SiHaNumb− tumors were established by subcutaneous injection of 1×107 cells in the right flank of mice. Tumor length and width were measured every 4 days and tumor volume (TV) was calculated using the following formula: TV = 0.5 × length × width2. At the end of the experiment, mice were sacrificed. Solid tumors were removed and processed for immunohistochemical analysis. Mice were killed by cervical dislocation after deep anesthesia with 2% isoflurane. All animal work was approved by Sichuan Animal Care (Chengdu, China). All mice were treated humanely throughout the experimental period.
Western blot analysis
For Western blotting analysis, the cells and samples were harvested, and then resuspended in RIPA. After incubation on ice for 1 h, the cell lysates were centrifugated for 15 min at 15,000 ×g. The protein concentration was determined by BSA protein assay kit and ~50 µg protein was applied to 10% SDS-PAGE gels and incubated with antibodies including Numb, Nothc1, Hes1, Shh and Smo at 4 °C overnight. Blots were developed with HRP-conjugated secondary antibodies and the protein bands were visualized using a chemiluminescence (ECL) detection system.
Immunofluorescence determination
Based on the results of Western blotting, we further confirmed the expression of Numb protein in cervical epithelial tissues at different pathological stages by immunofluorescence histochemical staining.
To assess the Numb level in mice tumor, we investigated Numb staining by the labeled streptavidin-biotin method. The primary antibody was rabbit anti-mouse polyclonal antibody Numb (BD, USA) and the secondary antibody was biotinylated goat anti-rabbit immunoglobulin (Abcam, USA).
Following the Immunofluorescent analysis protocol, frozen sections 4–5 µm thick of tumor tissue were fixed in cold acetone, washed with PBS, stained with Numb (1:200, BD, USA) overnight at 4 °C, washed twice with PBS, and incubated with a Cy3-conjugated second antibody (1:1,000, Abcam, USA) for 40 min. Toto-3/DAPI was added to stain nucleus (1:500). In the Numb staining tests, all the five tumors in each group were sectioned. In tumor tissue sections, five equal-sized fields were randomly chosen and analyzed. The expression of Numb was evaluated by calculating the average number of small Numb-positive cells (red) in five randomly selected tumor areas in each tumor sample by two independent investigators in a blinded fashion under a fluorescence microscopy.
Wound healing, Transwell invasion assay
The wound healing assay was performed using SiHa and SiHaNumb− cells. SiHa and SiHaNumb− were wounded by scratching with pipette tips and washed with PBS. After 24 h, images were taken by an Olympus digital camera. The migrated cells were quantified by manual counting, and the percentage of inhibition was expressed using untreated cells at 100%.
Transwell invasion assay was conducted as described previously with some modifications. The filter of the Transwell plate (Millipore) was coated with 50 µL Matrigel (BD Bio-sciences). After Matrigel polymerization, the bottom chambers were filled with DMEM medium containing 20% FBS and the top chambers were seeded with 100 µL DMEM medium (without FBS) and SiHa and SiHaNumb− (4×104 cells per well) and were allowed to migrate for 24 h. Cells on the upper side of the filter were removed. Cells located on the underside of the filter were fixed with methanol and stained with 0.5% crystal violet, then, migrated cells were quantified by manual counting and photographed under a light microscope. The percentage of migrated cells inhibition was expressed on the basis of medium-treated cells as control wells.
Preparation of SiHaNumb−
Short-hairpin RNAs (shRNAs) can be transduced to suppress gene function by RNA interference, which can model the impact of aberrant expression of target gene. By introducing shRNA targeting Numb into cervical squamous carcinoma cells SiHa, we can study the biological behavior alternation of SiHa. shRNA targeting Numb were linked to puromycin resistance, allowing cells transduced with shRNA to be selected by puromycin in vitro.
In vitro proliferation analysis
The SiHa and SiHaNumb− cells were analyzed by EdU detection kit to detect the cell proliferation. All experiments were performed at least in triplicate.
Statistical analysis
Statistical analysis was performed by one-way ANOVA unless otherwise specified. Significant differences between groups were indicated by *P<0.05, **P<0.01 and ***P<0.001.
Results
The Numb expression is different in tumor and normal cells
We first investigated the expression of Numb in squamous carcinoma of cervix cell lines SiHa, and non-tumorigenic keratinocytes HaCaT by western blot (Figure 1A) and immunofluorescence (Figure 1B). As shown, the Numb expression is obviously lower in SiHa than it in the HaCaT cells. Interestingly, the Numb expression in the HaCaT cells was well distributed (Figure 1B), while the expression in SiHa nucleus decreased significantly which was mainly detected in cell membrane and cytoplasm (Figure 1B).

Numb suppression endowed malignant features of cervical squamous carcinoma cells in vitro
Because of the different Numb expression between SiHa and HaCaT cells, we want to identify how Numb influence the cell proliferation, migration and invasion. We used RNA interference to characterize Numb functions in vitro. In this approach, various genetic elements are introduced into cervical squamous carcinoma cells SiHa by lentiviral mediated gene transfer. We obtained SiHaNumb− cells verified by the protein expression of Numb and observed a striking suppression of Numb on SiHaNumb− cells (Figure 1C). Subsequently, we investigated the alteration of biological behavior including cell proliferation, migration and invasion. The SiHaNumb− cells displayed higher cell proliferation rate than SiHa cells as shown using EDU assay (Figure 1D, *P<0.05). Meanwhile, as shown in Figure 1E-1H, SiHa Numb− cells exhibited enhanced invasion and migration by Transwell test and wound healing assay. These observations indicated that SiHa Numb− cells expressed malignant traits in response to Numb suppression compared with SiHa cells, in terms of enhanced cell proliferation, migration and invasion (*P<0.05).
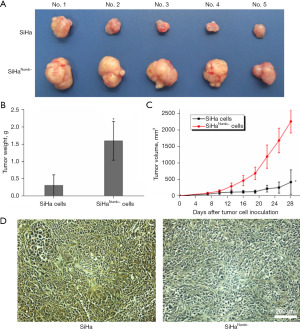
Numb suppression aggravated tumor malignance progression in xenograft mouse model in vivo
To investigate the role of Numb in tumor progression in vivo, we transplanted equal numbers of cervical squamous carcinoma cells SiHa or SiHaNumb− into the back flanks of nude mice and monitored their growth. All mice were sacrificed at day 28, whereas group with SiHaNumb− cells increased tumor size and weight, with a growth enhancement compared with SiHa groups in vivo (Figure 2A-2C). Immunohistochemistry analysis showed that the expression of Numb in malignant tumors formed by SiHaNumb− cells was lower (*P<0.05) (Figure 2D). Collectively, our observations indicated that suppression of Numb in SiHa cervical squamous carcinoma cells dramatically accelerated tumor growth and Numb supposedly function as tumor suppressor gene.
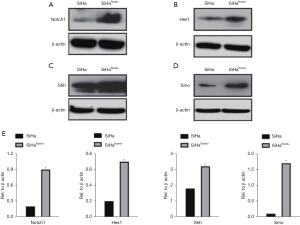
Suppression of Numb upregulated Notch1, Hes1, Shh and Smo expression
The aforementioned findings prompted us to investigate how Numb suppression in tumors accelerated malignant progression. To test this, we examined using by western blot the protein levels of components involved in tumor-related pathways. As shown in Figure 3A,3B, depletion of Numb in cervical squamous carcinoma cells SiHa resulted in a statistically significant increase of Notch1 and Hes1 protein compare with SiHa cells. Interestingly, a similar effect on Shh and Smo were observed after Numb depletion (Figure 3C-3E, *P<0.05).
Correlations between Numb expression and clinicopathological characteristics
To further assess the clinical relevance of our findings in cervical cancer, we investigated the Numb expression of clinical samples collected from patients suffering from cervical cancer with different clinicopathological characteristics. CIN is an intraepithelial neoplasia of the uterus and classified from CINI to CINIII grades by histology characteristics which represent a transitional stage from normal tissue to tumors of cervical squamous carcinoma. Therein, the grade of CINIII is severe dysplasia with undifferentiated neoplastic cells, also be referred to as cervical carcinoma in situ. In comparison with normal and CIN III group, the Numb expression in cervical squamous carcinoma was significantly lower than that in the two groups (Figure 4A). As shown in comparison of Numb expression between normal group, CIN group and cervical squamous carcinoma group, statistical analysis indicated that Numb protein was reduced in tumors compared with normal tissues and the expression of Numb gradually decreased from normal group to tumor group by western blot (Figure 4B). As shown in the comparison of Numb expression between cervical squamous carcinomas with different clinical stages, the expression of Numb protein in stage IIB and above was significantly lower than that in group I–IIA, and the difference was statistically significant (*P<0.05) (Figure 4C). In addition, as shown in comparison of Numb expression between cervical squamous carcinomas with different pathological grades, from high differentiation to poor differentiation, the expression of Numb gradually decreased with the increase of pathological grade. There was a statistically significant difference in the expression of Numb between the three groups (*P<0.05). Compared with the highly differentiated group, the moderately differentiated group and the poorly differentiated group, the expression of Numb was significantly decreased, and the difference was statistically significant (*P<0.05). Similarly, immunofluorescence revealed that the levels of Numb protein gradually decreased with the increase of pathological grade (Figure 4D). As shown in comparison of Numb expression between cervical squamous carcinomas with different lymph node metastasis, the expression of Numb in the lymphatic metastasis group was significantly lower than that in the no lymph node metastasis group (*P<0.05) (Figure 4E). The clinical data indicated low expression level of Numb may be a significant putative predictor to the prognosis of cervical squamous carcinomas.

Discussion
The study of the relationship between Numb and cancers was initiated by the P. P. Di Fiore laboratory. During embryonic development, Numb and Notch played antagonistic roles in asymmetric cell division, and enhanced Notch signaling pathways had been shown to be associated with tumorigenesis (36). Therefore, the Numb-Notch axis may be one of the causes of tumor genesis.
Previous studies found that the expression of Numb decreased in many tumors (37-39). These findings indicated that lack of Numb expression was closely related to tumor genesis or progression. In breast tumors, deletion of Numb resulted in excessive activation of Notch and inhibition of TP53 activity, both of which are responsible for the transformation. Thus, Numb was a tumor suppressor in breast cancer, and its functional inhibition promoted tumorigenesis by activating the oncogenic pathway (Notch) and inhibiting the tumor suppressor pathway (TP53) (40,41). In non-small cell lung cancer, Numb expression was also frequently lost, and similarly activation of the Notch pathway can also be observed. In this case, the inability to demonstrate a correlation with prognosis may be due to the confounding influence of additional, Numb-independent mechanisms causing Notch activation in these tumors, which was also supported by the fact that poor prognosis was associated with Notch activation (42). In breast and non-small cell lung cancers, loss of Numb expression due to exaggerated ubiquitination and ensuing degradation, despite the absence of genetic alterations in the NUMB locus (29,42). In these tumors, the genetic lesions upstream of Numb are still unknown. It may be present in a component of the ubiquitination/de-ubiquitination machinery, although studies of E3-ligands in breast cancers had not found alterations immediately consistent with the Numb status of these cancers. It had been shown that reduced Numb levels was also associated with poor prognosis in salivary gland cancers, although the exact mechanism of these tumors was unknown (22). It was further found that Numb was methylation by lysine methyltransferase Set8, which led to the increased Numb-p53 ubiquitination degradation and affected apoptosis ability (38). As a component of adhesion junction, Numb can also regulate cell adhesion and migration and participates in the ubiquitination of p53 and Gli1 (the main effector of Hedgehog signal), this affected the stability of GLI1 and inhibited Hedgehog signaling through a mechanism very similar to that of Notch signaling attenuation in mammals. In addition, Numb, expressed in granulocyte progenitors, inhibited the Hedgehog pathway, leading to its differentiation. As the mechanism between Notch and Hedgehog involves normal developmental programs and tumorigenesis, Numb may serve as a master regulator of both basic and closely integrated morphogenetic systems (43,44).
Previous studies have reported that Numb may play a role in tumorigenesis. However, the possible roles of Numb in the progression of cervical cancer have rarely been investigated. In this research, we found that a decrease in Numb was associated with malignant features of cervical squamous carcinoma, including malignant proliferation, migration and invasion in vitro, and greatly accelerated the progression of cervical squamous carcinoma in an in vivo xenograft mouse model (Figures 1,2). Mechanistic studies revealed that Numb inhibition stimulated Notch- and Hedgehog-dependent malignant transformation and promoted the progression of cervical squamous carcinoma (Figure 3). Clinical evidence also supported the involvement of loss of Numb function in the progression of cervical squamous carcinomas (Figure 4). Our findings identified Numb reduction as an important contributor to cervical cancer progression, rationalizing low expression level of Numb may be a predictor to the prognosis of cervical squamous carcinomas.
Conclusions
In summary, we proposed that the low expression of Numb promoted cervical squamous carcinoma cells proliferation, migration and invasion, greatly accelerated the progression of cervical squamous carcinoma in a xenograft mouse model. Furthermore, our data also showed that Numb inhibition promoted malignant transformation of cervical squamous carcinoma through stimulation of Notch- and Hedgehog-dependent pathways. What’s more, clinical evidence also supported that loss of Numb function promoted the progression of cervical squamous carcinoma. Our findings provided a new mechanism for understanding the deterioration of cervical squamous carcinoma cells and helped develop therapeutic strategies for treating cervical carcinoma.
Acknowledgments
Funding: This study was supported by the National Key Research and Development Program of China (No. 2021YFC2009100), the National Natural Science Foundation of China, Beijing, China (Grant No. 81101991) and the National Key Research and Development Program of China (No. 2020YFC2005600).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-30/rc
Data Sharing Statement: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-30/dss
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-23-30/coif). X.N. serves as the unpaid Executive Editor-in-Chief of Gynecology and Pelvic Medicine. T.C. serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from July 2023 to June 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Medical Ethics Committee of West China Second Hospital of Sichuan University (No. 2020126) and informed consent was obtained from all individual participants. All animal experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals, approved by the Ethics Committee of Sichuan University (Chengdu, China).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Basu P, Malvi SG, Joshi S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol 2021;22:1518-29. [Crossref] [PubMed]
- Duggan C, Trapani D, Ilbawi AM, et al. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol 2021;22:1632-42. [Crossref] [PubMed]
- Wang J, Wu Y, Li Y, et al. Guanosine monophosphate synthase upregulation mediates cervical cancer progression by inhibiting the apoptosis of cervical cancer cells via the Stat3/P53 pathway. Int J Oncol 2021;58:3. [Crossref] [PubMed]
- Dejima M, Hashimoto H, Sasajima Y, et al. Uterine cervical squamous cell carcinoma with reactive multinucleated giant cells expressing cluster of differentiation 204: A case report and literature review. J Obstet Gynaecol Res 2020;46:2174-8. [Crossref] [PubMed]
- Kato K, Terauchi M. Annual report of the Women's Health Care Committee, Japan Society of Obstetrics and Gynecology, 2020. J Obstet Gynaecol Res 2021;47:52-62. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543:378-84. [Crossref] [PubMed]
- Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. [Crossref] [PubMed]
- Melamed A, Margul DJ, Chen L, et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med 2018;379:1905-14. [Crossref] [PubMed]
- Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889-99. [Crossref] [PubMed]
- Scarth JA, Patterson MR, Morgan EL, et al. The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation. J Gen Virol 2021;102:001540. [Crossref] [PubMed]
- Chuerduangphui J, Pientong C, Swangphon P, et al. Association of antibody to E2 protein of human papillomavirus and p16(INK4A) with progression of HPV-infected cervical lesions. Med Oncol 2018;35:93. [Crossref] [PubMed]
- Goodman A. HPV testing as a screen for cervical cancer. BMJ 2015;350:h2372. [Crossref] [PubMed]
- Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010;316:900-6. [Crossref] [PubMed]
- Xu L, Li C, Hua F, et al. The CXCL12/CXCR7 signalling axis promotes proliferation and metastasis in cervical cancer. Med Oncol 2021;38:58. [Crossref] [PubMed]
- He C, Mao D, Hua G, et al. The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med 2015;7:1426-49. [Crossref] [PubMed]
- Jiang X, Xing H, Kim TM, et al. Numb regulates glioma stem cell fate and growth by altering epidermal growth factor receptor and Skp1-Cullin-F-box ubiquitin ligase activity. Stem Cells 2012;30:1313-26. [Crossref] [PubMed]
- Warowicka A, Wołuń-Cholewa M, Kwaśniewska A, et al. Alternations in mitochondrial genome in carcinogenesis of HPV positive cervix. Exp Mol Pathol 2020;117:104530. [Crossref] [PubMed]
- Katoh M, Katoh M. NUMB is a break of WNT-Notch signaling cycle. Int J Mol Med 2006;18:517-21. [Crossref] [PubMed]
- Kim KK, Nam J, Mukouyama YS, et al. Rbfox3-regulated alternative splicing of Numb promotes neuronal differentiation during development. J Cell Biol 2013;200:443-58. [Crossref] [PubMed]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell 2008;132:583-97. [Crossref] [PubMed]
- Paskeh MDA, Mirzaei S, Gholami MH, et al. Cervical cancer progression is regulated by SOX transcription factors: Revealing signaling networks and therapeutic strategies. Biomed Pharmacother 2021;144:112335. [Crossref] [PubMed]
- Maiorano E, Favia G, Pece S, et al. Prognostic implications of NUMB immunoreactivity in salivary gland carcinomas. Int J Immunopathol Pharmacol 2007;20:779-89. [Crossref] [PubMed]
- Mboumba Bouassa RS, Prazuck T, Lethu T, et al. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. Expert Rev Anti Infect Ther 2017;15:613-27. [Crossref] [PubMed]
- Feng X, Zhang D, Li X, et al. CXCL5, the upregulated chemokine in patients with uterine cervix cancer, in vivo and in vitro contributes to oncogenic potential of Hela uterine cervix cancer cells. Biomed Pharmacother 2018;107:1496-504. [Crossref] [PubMed]
- Molinolo AA, Marsh C, El Dinali M, et al. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin Cancer Res 2012;18:2558-68. [Crossref] [PubMed]
- Nieber F, Hedderich M, Jahn O, et al. NumbL is essential for Xenopus primary neurogenesis. BMC Dev Biol 2013;13:36. [Crossref] [PubMed]
- Gomez K, Ran D, Madura CL, et al. Non-SUMOylated CRMP2 decreases Na(V)1.7 currents via the endocytic proteins Numb, Nedd4-2 and Eps15. Mol Brain 2021;14:20. [Crossref] [PubMed]
- Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014;506:371-5. [Crossref] [PubMed]
- Pece S, Serresi M, Santolini E, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J Cell Biol 2004;167:215-21. [Crossref] [PubMed]
- Ishiyama S, Matsueda S, Jones LA, et al. Novel natural immunogenic peptides from Numb1 and Notch1 proteins for CD8+ cells in ovarian ascites. Int J Oncol 2007;30:889-98. [Crossref] [PubMed]
- Petersen PH, Zou K, Hwang JK, et al. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature 2002;419:929-34. [Crossref] [PubMed]
- Qian W, Hong Y, Zhu M, et al. Deletion of Numb/Numblike in glutamatergic neurons leads to anxiety-like behavior in mice. Brain Res 2017;1665:36-49. [Crossref] [PubMed]
- Refaat T, Donnelly ED, Sachdev S, et al. c-Met Overexpression in Cervical Cancer, a Prognostic Factor and a Potential Molecular Therapeutic Target. Am J Clin Oncol 2017;40:590-7. [Crossref] [PubMed]
- Dumont NA, Wang YX, von Maltzahn J, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 2015;21:1455-63. [Crossref] [PubMed]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 1994;76:477-91. [Crossref] [PubMed]
- Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstet Gynecol 2016;28:4-10. [Crossref] [PubMed]
- Schiffman M, Wentzensen N, Wacholder S, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 2011;103:368-83. [Crossref] [PubMed]
- Shan GP, Zhang P, Li P, et al. Numb Gene Enhances Radiation Sensitivity of Nonsmall Cell Lung Cancer Stem Cells. Cancer Biother Radiopharm 2016;31:180-8. [Crossref] [PubMed]
- Shu Y, Xu Q, Xu Y, et al. Loss of Numb promotes hepatic progenitor expansion and intrahepatic cholangiocarcinoma by enhancing Notch signaling. Cell Death Dis 2021;12:966. [Crossref] [PubMed]
- Verdi JM, Schmandt R, Bashirullah A, et al. Mammalian NUMB is an evolutionarily conserved signaling adapter protein that specifies cell fate. Curr Biol 1996;6:1134-45. [Crossref] [PubMed]
- Hounjet J, Vooijs M. The Role of Intracellular Trafficking of Notch Receptors in Ligand-Independent Notch Activation. Biomolecules 2021;11:1369. [Crossref] [PubMed]
- Westhoff B, Colaluca IN, D'Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009;106:22293-8. [Crossref] [PubMed]
- Tandon N, Thakkar KN, LaGory EL, et al. Generation of Stable Expression Mammalian Cell Lines Using Lentivirus. Bio Protoc 2018;8:e3073. [Crossref] [PubMed]
- Guo Y, Zhang K, Cheng C, et al. Numb(-/low) Enriches a Castration-Resistant Prostate Cancer Cell Subpopulation Associated with Enhanced Notch and Hedgehog Signaling. Clin Cancer Res 2017;23:6744-56.M
Cite this article as: Wang H, Bai L, Zhang W, He Y, Niu X, Cui T. Numb suppression promoted the pathogenesis of SiHa cells by stimulating Notch and Hedgehog signaling pathways. Gynecol Pelvic Med 2023;6:21.


