Cervical cancer in pregnancy: one case report and a review of current treatment recommendations
Introduction
Cervical cancer is the most commonly diagnosed gynecological malignancy during pregnancy mainly, and the majority is in stage I. Choosing between delaying treatment until their pregnancy reaches full term or opting for immediate treatment is often a difficult decision for both the patient and the doctor. Patients who choose to delay the therapy until the fetus becomes mature enough for birth should undergo a cesarean section, during which a radical hysterectomy plus pelvic lymphadenectomy can also be performed. If the patient does not continue the pregnancy, there are several treatment choices. For example, radiotherapy alone, surgery after radiotherapy, surgery alone, and others, could be made depending on the stage and the gestational week. For patients who choose radiotherapy, traditional radiotherapy with or without chemotherapy will need to be adjusted appropriately. Transvaginal radical hysterectomy has been reported in some patients with early cervical cancer.
Our current case was a pregnant woman with stage IB1 poorly differentiated squamous cell carcinoma of the cervix. After detailed discussions with multiple departments and adequate communication with the patient and her families, neoadjuvant chemotherapy was first applied, followed by radical hysterectomy plus pelvic lymphadenectomy during the cesarean section when the fetus becomes mature enough for birth.
Case presentation
A 37-year-old G8P3+4 pregnant woman was admitted to our hospital at 21+3 weeks of gestational age because of abnormal vaginal bleeding for one month, and a cervical lesion identified ten days ago.” She had received regular antenatal check-ups in another hospital. At 15 weeks of gestational age, she had a small amount of bright red vaginal bleeding without an apparent cause, which lasted for about ten or more days. No abdominal pain, fever, or other discomfort was noted. She received an antenatal examination in a local hospital, during which, the physical examination revealed a 2 cm neoplasm at the posterior lip of the cervix. HPV testing showed that HPV types 16 and 51 were positive. And a colposcopically directed cervical biopsy showed a cervical intraepithelial neoplasia grade 3 (CIN 3), and microinvasive squamous cell carcinoma was discovered in the local hospital. For more treatment, the patient visited the outpatient department in our hospital, and a revised pathological diagnosis of “CIN 3 involving glands” without microinvasive squamous cell carcinoma was made after consultation in the pathology department of our hospital.
For further treatment, she visited the gynecological outpatient clinic in our hospital in the 20th gestational week. After a gynecological examination, the doctor believed the CIN3 diagnosis was not consistent with her clinical manifestation, so a second biopsy under the colposcopy was undertaken. On September 12, 2016, the diagnosis of poorly differentiated squamous cell carcinoma was confirmed by the cervical samples from the second biopsy in our hospital. Then, the patient was admitted to the Gynecological Department. Gynecological examination showed the uterine height was 25 cm, and the abdominal circumference was 98 cm. The development of vulva was normal. Her vagina was unobstructed, without any deformity or abnormal mucosal color. The cervix was hypertrophic, with a 2-cm cauliflower-like neoplasm visible at its lower lip and the parametrium was soft. The lesion demonstrated contact bleeding; however, no bleeding was seen inside the cervical canal. Color Doppler ultrasonography revealed a single live fetus. The diagnoses were as follows: stage IB1 poorly differentiated squamous cell carcinoma of the cervix; G8P3+4 21+3 weeks of a single live fetus.
After discussions with multiple departments and adequate communication with the patient and her families about the benefits and risks of relevant treatment protocols, a treatment plan was established. The patient would first undergo neoadjuvant chemotherapy, followed by the termination of the pregnancy when the fetus becomes mature enough for birth. From September 18, 2016, to November 18, 2016, the patient received three cycles of TP (paclitaxel + cisplatin) intravenous chemotherapy in our hospital. Then she was regularly followed up in the gynecological and obstetrical departments, during which the disease did not aggravate obviously. On December 27, 2016, she received transabdominal cesarean section, radical hysterectomy, bilateral salpingectomy, bilateral ovarian transposition and pelvic lymphadenectomy under general anesthesia. The surgery was successful, and a live male baby was successfully delivered. The patient was discharged after seven days, and the abdominal incision healed well. The urinary catheter was kept for 49 days after the operation until the bladder function was recovered.
Postoperative pathological examination showed positive HPV16 and poorly differentiated squamous cell carcinoma of the uterus. Lymphovascular space invasion was determined, and the lesion had invaded 1/3 of cervical stroma, without involving the cervical junction, vaginal dome, surgical stump, left and right parauterine, resection margin of left and right pelvic wall, or left and right fallopian tubes. Metastasis was identified in one of four right pelvic lymph nodes, and reactive hyperplasia was found in 8 left pelvic lymph nodes (no carcinoma metastasis was found). Endometrial changes were typical during pregnancy, and chronic inflammation was noted in left and right fallopian tubes. Immunohistochemistry (IHC) showed that P16, +++; P63, ++; CK5/6, ++; Ck18, −; Ck8, −; CK7, −, and the fractions of Ki-67-positive tumor cells was about 80%. The patient received an additional 35 sessions of radiotherapy in another hospital. No recurrence was found in the follow-up period (the last follow-up visit was on January 14, 2019). The neonate had a good prognosis; his development was normal, and there was no special condition.
iMDT discussion
Discussion among physicians from the West China Second University Hospital of Sichuan University
Cervical cancer is the most commonly diagnosed gynecological malignancy during pregnancy, and the majority is in stage I. The frequency of cervical cancer is about 1 to 2 cases in 2,000 to 10,000 pregnancies (1,2). About 0.5% to 3% of cases of cervical cancer are diagnosed during pregnancy. In 2018, the Chinese Society of Obstetrics and Gynecology suggested that cervical cytology should be listed as a necessary pre-conceptional and prenatal examination (3). It had been proposed that the survival rate of pregnant women with cervical cancer is relatively lower as compared to the non-pregnant females; however, most studies have found that the prognosis has not been affected (4).
At present, there is no consensus on the treatment protocol of cervical cancer during pregnancy, the timing of delivery, and the mode of delivery. Delay of treatment until the pregnancy reaches full term or receiving an immediate remedy is often a difficult decision for both patients and doctors. According to the International Gynecologic Cancer Society (IGCS) and the European Society of Gynecological Oncology (ESGO), for patients who do not want to continue with their pregnancy, the treatment is the same as for non-pregnant women. For women deciding to continue with the pregnancy, individualized treatment should be applied, the timing of termination of pregnancy and the treatment protocol of cervical cancer can be determined according to disease stage and gestational age (5,6). Multiple factors including gestational age, tumor stage, metastasis, the wishes of the pregnant woman and her families, and the expectation of the pregnancy outcome should be taken into account when choosing the treatment option.
In 2018, the Chinese Anti-Cancer Association (CACA) released recommendations on the treatment of cervical cancer in pregnancy (7). As for the patients who want to continue the pregnancy, the following recommendations should be considered.
- Patients with stage IA1 cervical cancer should be closely monitored. Colposcopy should be performed every eight weeks, and cervical biopsy may be performed if necessary. The treatment could be initiated postpartum if the disease is not progressive, but the possible risk about the progression of disease should be informed to the patient (7).
- For patients with stage IA2–IV cervical cancer within the first 20 weeks of gestation, continuing the pregnancy is not recommended. In the early trimester, the fetus is often resected together with the uterus (2).
- For patients with stage IA2–IB1, cervical cancer sized <2 cm with negative lymph node involvement, simple trachelectomy or extended cervical conization can be performed if the diagnosis is confirmed within 25 weeks of gestation. But the radical trachelectomy was not advised because of the high possibility of abortion after surgery (8). For patients with stage IA2–IB1, cervical cancer sized <2 cm after 25th gestational week, metastasis status of the lymph node is difficult to confirm because of the enlarged uterus, neoadjuvant chemotherapy or close monitoring antepartum and delayed standard treatment postpartum is a choice of treatment (9).
- The management of patients with cervical cancer in the second trimester remains controversial. The CACA’s suggestion is as follows: for patients with stage IA–IIA cervical cancer diagnosed during the second trimester (20–28 weeks) and have strong desires to continue the pregnancy, pregnancy may be continued with close monitoring. For patients with cervical cancer later than stage IIB, keeping the pregnancy is not recommended, but the desire of the patient about pregnancy should be respected, and the risk should be analyzed (7). If the patients have strong ambitions, neoadjuvant chemotherapy may be applied to retain the fetus until fetal maturity, as proposed by a European consensus (6). Compared to fetal exposure to chemotherapy, extremely preterm birth is associated with a far higher risk of poor fetal survival and outcome (10). According to IGCS and ESGO (5,6), if the diagnosis for patients with stage IA1–IB1 cervical cancer is confirmed after the 25th gestational week, neoadjuvant chemotherapy (NACT) can be used to continue the pregnancy until the fetal lung matures.
- For pregnant patients with locally advanced and metastatic cervical cancer (stages IIB–IV), it is typically recommended that the pregnancy should be immediately terminated to initiate the treatment of cervical cancer. The standard treatment for locally advanced cervical cancer is radiotherapy and chemotherapy. Usually, therapeutic doses of pelvic EBRT cause fetal demise (5). The management of patients with cervical cancer later than stage IIB in the second trimester remains controversial. Risks should be fully assessed, and the patients’ choices should be respected. If the patients have strong desires, neoadjuvant chemotherapy may be applied to retain the fetus until fetal maturity, as proposed by a European consensus (6).
The mode of delivery for pregnant women with cervical cancer has been controversial. For young pregnant women with early invasive cervical cancer (stages IA2, IB, and IIA), the fetus is often removed together with the uterus in the first trimester; in the third trimester, a classical cesarean section can be performed firstly, followed by radical hysterectomy and lymph node dissection (3). For pregnant women with locally advanced cervical cancer, radiotherapy and chemotherapy are usually applied in the first trimester. Most patients will experience spontaneous abortion within 2 to 5 weeks from the beginning of radiation. In the late first trimester, the spontaneous abortion rate decreases. Some patients need to receive complete curettage of the uterine cavity at the end of external beam radiotherapy. For patients with cervical cancer in the second and third trimesters, a classical cesarean section can be performed firstly, followed by standard radiation and chemotherapy. A vaginal birth may be considered for patients with small stage IA1 lesions (11). For large and hard bulky-barrel lesions or large, vulnerable, bleeding exophytic lesions, the risk of vaginal delivery is exceptionally high. Studies have shown that compared with stage I cervical cancer, stage II A or above cervical cancer had higher recurrence rate and lower survival rate after vaginal delivery, and lateral episiotomy at vaginal delivery increased the implantation and metastasis of tumors (12). Therefore, for pregnant women with stage I A2 or later-staged cervical cancer above, the risk of complications such as obstructed labor, hemorrhage, sepsis, cervical laceration, dissemination of disease into lymphovascular channels, and tumor implantation at episiotomy site can be high, cesarean section is recommended for these patients (13). The 2018 Chinese Expert Consensus on the Management of Cervical Cancer in Pregnancy and the Guidelines on Diagnosis and Treatment of Cervical Cancer (7,8) propose that, when cesarean section for pregnancy with cervical cancer is performed, any possible placenta metastasis should be carefully examined during the operation.
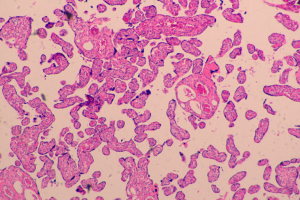
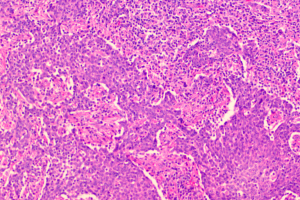
Our current patient had stage IB1 cervical cancer during pregnancy, and the patient and her family members requested to continue the pregnancy. After consultations with multiple departments, three cycles of neoadjuvant chemotherapy using TP regimen was performed. After the fetal lung matured in the 34th gestational week, cesarean section was completed, along with radical hysterectomy and pelvic lymphadenectomy. No placental metastasis was found in postoperative pathology (Figures 1,2). The prognosis of the mother and the infant were perfect.
What’s the optimal treatment protocol for stage IIB and above cervical cancer in the second, and third trimesters of pregnancy if the patient wanted to terminate the pregnancy?
Expert opinion 1: Junjie Wang, Michael D. Mueller, Andrea Papadia
The standard treatment for patients in this group is chemo-radiation therapy. In a systematic review and meta-analysis, Green et al. confirmed the superiority of chemo-radiation over radiation alone. Concurrent chemotherapy and radiotherapy improves overall and progression-free survival and reduces local and distant recurrence in selected patients with cervical cancer (14).
Uterine evacuation, in the form of suction evacuation or hysterotomy, is usually performed before starting treatment. The dose and regime of chemo-radiation are the same as the usual setting.
Expert opinion 2: Linda Mileshkin
The optimal treatment protocol should be the same as that recommended for a non-pregnant patient after the surgery has been terminated. For locally advanced cervix cancer, this would usually involve treatment with concurrent chemoradiation. The termination of pregnancy after the first trimester usually requires a cesarean section as vaginal delivery is not safe in this instance due to the potential risks of blood loss or scar recurrence at the site of episiotomy (15).
What’s the optimal treatment protocol for stage IIB and above cervical cancer in the second, and third trimesters of pregnancy if the patient wanted to continue the pregnancy?
Expert opinion 1: Junjie Wang, Michael D. Mueller, Andrea Papadia
General comments
Treatment of cervical cancer in pregnancy is dependent on the stage of the disease, gestational age, histological subtype, and patient's wish to continue the pregnancy. These patients should be managed in a tertiary center under a multidisciplinary team, consisting of gynae-oncologists, obstetricians, neonatologists, and oncologists. Evaluation of the tumor is based on clinical examination and magnetic resonance imaging. The use of magnetic resonance imaging for the assessment of local extension and lymph nodal status is safe and effective. A chest X-ray can be performed after the first trimester with a lead shield. The experimental nature of cancer treatment in pregnancy and the risks involved should be informed to the patient. They should be reminded that chemo-radiation is the recommended treatment for locally advanced cervical cancer. In a single-center, phase III, randomized controlled trial, Gupta et al. showed that cisplatin-based concomitant chemo-radiation has superior disease-free survival compared with neoadjuvant chemotherapy (NACT) followed by radical surgery in locally advanced cervical cancer (16).
In patients with an aggressive histology
In patients with a more aggressive histological subtype like small cell carcinoma of the cervix, preservation of pregnancy is not recommended, and standard treatment should be commenced as soon as possible.
In patients who are near term or at term
For patients at term, a cesarean delivery is advised, followed by standard treatment.
In patients with pregnancy before viability
Interruption of pregnancy is recommended if the fetus is not viable.
In patients with viable fetus before the term
Use of NACT in locally advanced cervical cancer in pregnancy is reported in several case reports and series. However, the use of NACT in cervical cancer in pregnancy has not been adequately assessed because of its rarity, the difficulty for long-term follow up of exposed children and unrealistic to conduct a randomized controlled trial. The risk of progression of the disease, with worsening prognosis, while on NACT, should be explained to the patient.
NACT allows the pregnancy to reach a good gestation (34–35 weeks) before cesarean delivery and hence reduce the risks associated with prematurity. It can reduce the size of the tumor and prevent dissemination of the disease until fetal maturity. The choice of chemotherapy should be platinum-based, and addition of paclitaxel may increase response rates (17,18). The TIP (paclitaxel, ifosfamide, cisplatin) regimen is considered the most effective NACT treatment according to the SNAP01 trial (19). It is associated with a higher response rate than the IP (ifosfamide and cisplatin) regimen. However, as ifosfamide is toxic to fetal kidneys, it cannot be used during pregnancy.
The chemotherapy is administered every 3 weeks. Delivery is planned at least 3 weeks after the last cycle of chemotherapy to reduce the risk of bleeding, infection, and anemia in both mother and child. Chemotherapy is contraindicated in the first trimester and should not be given after 35 weeks of gestation. Standard treatment of chemo-radiation should be administered after delivery. Radical surgery can be considered at the time of the cesarean section if the tumor has responded well to the NACT.
Expert opinion 2: Taek-Sang Lee
The optimal management of cervical cancer in pregnancy has not been determined because we have no published randomized trials. Therefore, the strategy should depend upon gestational age at diagnosis, stage of the disease, lymph nodes involvement, patient’s desire about her actual pregnancy. The definition of the second trimester varies slightly depending on literature, but usually starts in week 13 or 14 of pregnancy and lasts through the end of 26 or 27 weeks.
In stage IIB disease, if the patient who is in early second trimester wanted to terminate the pregnancy, primary chemoradiotherapy should be considered. Then, we may have two options—radiotherapy after uterine evacuation or radiotherapy with the fetus in utero. In the former option, however, the size of the tumor made dilatation and evacuation not feasible due to potential life-threatening procedure-related hemorrhage, but that does not mean that evacuation of the pregnancy by hysterotomy should be chosen because chemoradiation immediately after hysterotomy would interfere with wound healing and thus waiting for complete wound healing would result in delay of treatment. Even so, medical termination using misoprostol cannot be considered as an option due to the presence of bulky cervical lesions. Therefore, the latter option would be preferred in this situation. Spontaneous abortion occurs within 4–6 weeks after the initiation of radiotherapy but, in some case report, we may need to consider moral distress of radiation oncologists over the use of radiation treatment to the viable fetus. Therefore, fetocide by fetal intra-cardiac potassium chloride injection before initiation of radiation therapy can be discussed. After successful shrinkage of the primary lesion is achieved, elective labor induction may be considered in the middle of radiation treatment to avoid a risk of coagulopathy or infection.
If the patient strongly wanted to continue the pregnancy, the gestational age at diagnosis would be the most critical factors to the decision. If the patient is in the late second or third trimester, options of pregnancy continuation or termination were discussed with the patient thoroughly, including the risk of rapid tumor progression. Then, neoadjuvant chemotherapy can be cautiously considered.
If the patients were in the early second trimester, it would better to discourage the patient wishes to preserve pregnancy considering the risk of tumor progression and potential adverse fetal outcome related with a relatively long exposure of chemotherapeutic agent.
Expert opinion 3: Linda Mileshkin
Management of such patients needs to be multi-disciplinary and involve both the obstetric and oncology team. It should occur in centers with appropriate expertise and resources to manage pregnant patients with cancer. The patient requires both psychosocial support but also careful counseling so that she understands the potential risks to both herself and her child of continuing with the pregnancy.
Some investigations to determine the extent of cancer can be safely performed during pregnancy, such as the use of chest X-ray with abdominal shielding, ultrasound and the use of MRI without Gadolinium (which is not safe to use in pregnancy). PET/MRI if available, can also be safely performed and would provide an optimal assessment of the nodal status. The use of neoadjuvant chemotherapy during the second and third trimesters can be considered to manage cancer up until the time that delivery is planned. This is usually at around 37 weeks to optimize the chances of excellent fetal outcomes. Chemotherapy would usually involve 3–4 cycles of carboplatin and paclitaxel chemotherapy. Carboplatin is preferred over cisplatin because of the risks of ototoxicity in the fetus if cisplatin is used. It will be noted that the calculated GFR will be very high during pregnancy, but carboplatin can be safely dosed to the calculated value. Had the patient been found to have metastatic disease, then the use of bevacizumab during pregnancy would not be recommended. The last cycle of chemotherapy should be planned to occur 3 weeks before the scheduled delivery date. Routine anti-emetics, such as metoclopramide and ondansetron, as well as steroid pre-medication with chemotherapy, can be used. However, some recommend that the use of hydrocortisone or prednisolone is preferred over prednisolone as they are extensively metabolized in the placenta, and hence will result in little fetal exposure. The delivery needs to be done by cesarean section as discussed earlier. Following delivery, standard treatment with chemoradiation can be given. It is recommended to wait 6 weeks for the uterus to involute before commencement. It will not be possible for the mother to breastfeed during chemotherapy treatment due to potential transmission to the child (15).
What’s the operational difficulties and precautions for the surgery of cervical cancer in pregnancy?
Expert opinion 1: Junjie Wang, Michael D. Mueller, Andrea Papadia
In the presence of a tumor on the cervix, cesarean section is the preferred route of delivery. Vaginal delivery may result in the lymphovascular spread, bleeding, obstruction of the birth canal, laceration of vagina and cervix and implantation of malignant cells on episiotomy wound. Abdominal wall recurrences have also been described after surgery, and hence a wound protective system can be used during cesarean section. As the cervical tumor is vascular, it is best to avoid the tumor by performing a classical incision of the uterus for delivery of the fetus. The method for radical hysterectomy, parametrial dissection, and ureteral mobilization is similar to a non-pregnant uterus, despite a larger uterine size. Particular precaution should be made to control hemostasis, as blood vessels will remain engorged.
Cross-matched blood should be readily available. Experienced gynae-oncologists in a tertiary center should perform the surgery.
Expert opinion 2: Taek-Sang Lee
When confined to radical hysterectomy, the significant changes of the uterus in pregnancy such as the larger uterine volume and increased uterine blood flow do not significantly affect the performance in the dissection of the parametria, ureteral mobilization and do not differ considerably in blood loss from those observed among non-pregnant women (20). Also, pelvic lymphadenectomy in patients with early invasive cervical cancer during the first and second trimester is a safe and reproducible operation with good oncological outcomes with minimal surgery-related complications (21).
What’s the potential impact of high-risk HPV to the male and female fetus antepartum and postpartum?
Expert opinion 1: Junjie Wang, Michael D Mueller, Andrea Papadia
Vertical transmission of HPV from mother to fetus is known to occur. Up to 80% of neonates born to women with genital HPV have HPV DNA detectable in their nasopharyngeal aspirate or oral mucosa (22), and this may persist for months or years. Despite a high prevalence of HPV DNA detection, the children are usually asymptomatic.
Perinatal transmission of HPV types 6 and 11 can lead to the development of juvenile-onset recurrent respiratory papillomatosis, but this is rare with an estimated annual incidence of two to four per 100,000 infants (23,24).
Expert opinion 2: Taek-Sang Lee
While the risk of vertical transmission has been reported to be relatively low, it is clear that High-risk HPV can be transmitted during the delivery process. A recent study reported that among 153 healthy mothers who have a positive HPV test in the antenatal period, 5.2% of neonates showed HPV DNA positive in their cord blood or nasopharyngeal aspirates (25). However, HPV persistence in infants is known to be a rare event, and the substantial HPV positivity can be observed in children born to HPV-negative mothers (26).
Expert opinion 3: Linda Mileshkin
Vertical transmission of HPV infection from the mother to the fetus has been described in multiple case series. Hence it has been recommended that the mother receives HPV vaccination during pregnancy to reduce the risk of fetal infection. Several series have now been published suggesting that HPV vaccination during pregnancy is safe (15).
What’s the impact of chemotherapy on fetal prognosis?
Expert opinion 1: Junjie Wang, Michael D Mueller, Andrea Papadia
The timing of exposure, the dose of the drug, and the degree to which the drug crosses the placenta influence the impact of chemotherapy on the fetus. First-trimester use of chemotherapy should be avoided as it increases the risk of fetal malformation, miscarriages, and fetal death (27). The use of chemotherapy in the second and third trimesters is associated with intrauterine growth restriction and low birth weight.
Several studies have proved that the use of chemotherapy in pregnancy is not associated with learning disabilities, hematological, or immunological abnormalities in exposed children. In a prospective study, Amant et al. found that the general health, cognitive development, and cardiac function of children who were exposed to chemotherapy in utero were comparable to children who did not (28).
Platinum derivatives were shown to be a safe option during pregnancy (29). Marnitz et al. measured cisplatin concentration in maternal blood, amniotic fluid, and umbilical cord blood of patients with cervical cancer in pregnancy. They found that the levels of cisplatin in umbilical cord blood and amniotic fluid are significantly lower than the levels found in the maternal blood. These results were confirmed by the same group of authors in another study on platinum agents (30,31). As cisplatin is the most common drug used in neoadjuvant chemotherapy treatment of cervical cancer in pregnancy, these results assure us of the safety of the drug.
It takes about 3 weeks for the bone marrow to recover after chemotherapy. Hence, delivery must be planned at least 3 weeks from the last cycle of chemotherapy, and chemotherapy should be avoided after 35 weeks of gestation. This will reduce the risk of infection and hemorrhage to both mother and child.
Expert opinion 2: Taek-Sang Lee
According to the evidence so far, exposure to chemotherapy in the second or third trimester of pregnancy is not detrimental to fetal life, but the risks of small for gestational age and preterm birth still exist. Cytotoxic chemotherapy administered during the first trimester has been known to be associated with an increased risk of congenital malformations and is therefore contraindicated.
Agents for targeted therapy such as bevacizumab has become an important therapeutic option for in metastatic disease of cervical cancer, but the data on the safety of these agents in pregnancy is overall scarce. Because of the small size, structure, metabolism, and pharmacokinetics of these agents, they are potentially teratogenic and harmful for the fetus (32) and these agents should, therefore not be advised in pregnancy; hormonal treatment in pregnancy is contraindicated.
Expert opinion 3: Linda Mileshkin
The impact of chemotherapy on fetal prognosis depends on the drugs used as well as the timing of delivery. Several different review articles provide an excellent summary of this information such as that by Maggen et al. In general, chemotherapy use is contraindicated during the first trimester as use has been reported to be associated with spontaneous miscarriage, fetal death, and congenital malformations. In the second, and particularly the 3rd-trimester chemotherapy can be given, although there is a risk of the increased likelihood of complications such as intra-uterine growth restriction, stillbirth, preterm birth or need for neonatal intensive care. Data on pediatric outcomes are not extensive but generally, do not report a significant incidence of long-term complications in children exposed to chemotherapy when in-utero (15).
Conclusions
Treatment of cervical cancer in pregnancy is dependent on the stage of the disease, gestational age, histological subtype, and patient’s wish to continue the pregnancy. These patients should be managed under a multidisciplinary team. For patients who do not want to continue with their pregnancy, the treatment is the same as for non-pregnant women. For women deciding to continue with the pregnancy, individualized treatment should be applied. For pregnant patients with locally advanced and metastatic cervical cancer (stages IIB–IV) or with a more aggressive histological subtype like small cell carcinoma of the cervix, it is recommended that the pregnancy should be immediately terminated to initiate the treatment. For IA–IIA stage, different treatment including interruption of pregnancy, expectant management, neoadjuvant chemotherapy should be applied according to the gestational week.
The termination of pregnancy after the first trimester usually requires a classical incision of cesarean section as vaginal delivery is not safe because of the potential risks of blood loss in the tumor lesion during vaginal delivery. Vertical transmission of HPV from mother to fetus is usually harmless to the children. First-trimester use of chemotherapy should be avoided. The use of chemotherapy in the second and third trimesters is not associated with learning disabilities in the exposed children. This case could help surgeons have a better understanding and choosing about the treatment protocol of cervical cancer during pregnancy, the timing of delivery, and the mode of delivery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “iMDT Corner”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm.2019.07.01/coif). The series “iMDT Corner” was commissioned by the editorial office without any funding or sponsorship. XH serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Jun 2018 to May 2020. AP serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Jun 2018 to May 2020. PW serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Jun 2018 to May 2020. WJ serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from May 2019 to Apr 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCCN. Cervical Cancer Version 2 2019.NCCN Clinical Practice Guidelines in Oncology.
- Nguyen C, Montz FJ, Bristow RE. Management of stage I cervical cancer in pregnancy. Obstet Gynecol Surv 2000;55:633-43. [Crossref] [PubMed]
- Qi HB, et al. Guidelines on Preconceptional and Prenatal Care (2018 edition). Chin J Obstet Gynecol 2018;53:7-13.
- Gonçalves CV, Duarte G, Costa JS, et al. Diagnosis and treatment of cervical cancer during pregnancy. Sao Paulo Med J 2009;127:359-65. [Crossref] [PubMed]
- Amant F, Van Calsteren K, Halaska MJ, et al. Gynecologic cancers in pregnancy: guidelines of an international consensus meeting. Int J Gynecol Cancer 2009;19:S1-S12. [Crossref] [PubMed]
- Amant F, Halaska MJ, Fumagalli M. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer 2014;24:394-403. [Crossref] [PubMed]
- China Anti-Cancer Association Professional Committee on Gynecological Tumors. Guidelines on Diagnosis and Treatment of Cervical Cancer. Chin J Prac Gynecol Obstetr 2018;34:613-22.
- Wei LH, Zhao Y, Xie X, et al. Expert consensus cervical cancer in pregnancy. Chin J Clin Obstetrics Gyn Ecol 2018;19:190-2.
- Song KQ, Di W. Advances in the Diagnosis and Treatment of Pregnancy Complicated with Cervical Disease. J Int Obstet Gynecol 2017;44:356-60.
- Ilancheran A. Neoadjuvant chemotherapy in cervical cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol 2016;33:102-7. [Crossref] [PubMed]
- Han SN, Mhallem Gziri M, Van Calsteren K, et al. Cervical cancer in pregnant women: treat, wait or interrupt? Assessment of current clinical guidelines, innovations and controversies. Ther Adv Med Oncol 2013;5:211-9. [Crossref] [PubMed]
- Sood AK, Sorosky JI, Mayr N, et al. Cervical cancer diagnosed shortly after pregnancy: prognostic variables and delivery routes. Obstet Gynecol 2000;95:832-8. [PubMed]
- Van Calsteren K, Vergote I, Amant F. Cervical neoplasia during pregnancy: diagnosis, management and prognosis. Best Pract Res Clin Obstet Gynaecol 2005;19:611-30. [Crossref] [PubMed]
- Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781-6. [Crossref] [PubMed]
- Maggen C, van Gerwen M, Van Calsteren K, et al. Management of cancer during pregnancy and current evidence of obstetric, neonatal and pediatric outcome: a review article. Int J Gynecol Cancer 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2;IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J Clin Oncol 2018;36:1548-55. [Crossref] [PubMed]
- Moore KN, Herzog TJ, Lewin S, et al. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IVB, recurrent or persistent cervical cancer. Gynecol Oncol 2007;105:299-303. [Crossref] [PubMed]
- Mori T, Hosokawa K, Kinoshita Y, et al. Neoadjuvant chemotherapy with weekly carboplatin and paclitaxel for locally advanced cervical carcinoma. Int J Gynecol Cancer 2008;18:85-9. [Crossref] [PubMed]
- Buda A, Fossati R, Colombo N, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study. J Clin Oncol 2005;23:4137-45. [Crossref] [PubMed]
- Traen K, Svane D, Kryger-Baggesen N, et al. Stage Ib cervical cancer during pregnancy: planned delay in treatment—case report. Eur J Gynaecol Oncol 2006;27:615-7. [PubMed]
- Vercellino GF, Koehler C, Erdemoglu E, et al. Laparoscopic Pelvic Lymphadenectomy in 32 Pregnant Patients With Cervical Cancer. Int J Gynecol Cancer 2014;24:364-71. [Crossref] [PubMed]
- Rombaldi RL, Serafini EP, Mandelli J, et al. Perinatal transmission of human papillomavirus DNA. Virol J 2009;6:83. [Crossref] [PubMed]
- Levi JE, Delcelo R, Alberti VN, et al. Human papillomavirus DNA in respiratory papillomatosis detected by in situ hybridization and the polymerase chain reaction. Am J Pathol 1989;135:1179-84. [PubMed]
- Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope 2008;118:1236-47. [Crossref] [PubMed]
- Lee SM, Park JS, Norwitz ER, et al. Risk of Vertical Transmission of Human Papillomavirus throughout Pregnancy: A Prospective Study. PLoS One 2013;8:e66368 [Crossref] [PubMed]
- Castellsagué X, Drudis T, Cañadas MP, et al. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis 2009;9:74. [Crossref] [PubMed]
- Marnitz S, Schmittel A, Bolbrinker J, et al. The therapeutic management of a twin pregnancy complicated by the presence of cervical cancer, following laparoscopic staging and chemotherapy, with an emphasis on cisplatin concentrations in the fetomaternal compartments amnion fluid, umbilical cord, and maternal serum. Fertil Steril 2009;92:1748.e1-4. [Crossref] [PubMed]
- Amant F, Van Calsteren K, Halaska MJ, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol 2012;13:256-64. [Crossref] [PubMed]
- Zagouri F, Sergentanis TN, Chrysikos D, et al. Platinum derivatives during pregnancy in cervical cancer: a systematic review and meta-analysis. Obstet Gynecol 2013;121:337-43. [Crossref] [PubMed]
- Marnitz S, Köhler C, Oppelt P, et al. Cisplatin application in pregnancy: first in vivo analysis of 7 patients. Oncology 2010;79:72-7. [Crossref] [PubMed]
- Köhler C, Oppelt P, Favero G, et al. How much platinum passes the placental barrier? Analysis of platinum applications in 21 patients with cervical cancer during pregnancy. Am J Obstet Gynecol 2015;213:206.e1-5. [Crossref] [PubMed]
- Lambertini M, Peccatori FA, Azim HA Jr. Targeted agents for cancer treatment during pregnancy. Cancer Treat Rev 2015;41:301-9. [Crossref] [PubMed]
Cite this article as: Han J, Hu X, He X, Wang J, Mueller MD, Papadia A, Lee TS, Mileshkin L, Wang P, Yao Q, Jiang W. Cervical cancer in pregnancy: one case report and a review of current treatment recommendations. Gynecol Pelvic Med 2019;2:10.