Use of GnRH analogue in the endometriosis recurrence after surgery
Introduction
Endometriosis recurrence represents a fight for the gynecologist. The recurrence rate after surgery varies considerably in scientific literature due to multiple factors. Firstly, the definition of the recurrence is different among the available studies. Some studies consider “recurrence” the symptoms reported by patients, while others measure the recurrence with more objective clinical/instrumental methods, for instance, the assessment of endometriomas relapse by ultrasonography. Moreover, the length of follow-up varies among the sources, the previous surgical radicality and technique are different (1).
The general reported incidence rate of disease recurrence, regardless of the definition, is 19.1% after two years from surgical treatment and it ranges between 20% and 44% after five years (1-3). The subjective recurrence seems more frequent than the objective one and there is a low correlation between the symptoms and the stage of the disease. The objective rate is reported to be 9% after three years post-surgery and 28% after 5 years, while the symptoms recurrence varies between 20.5% after 3 years and 43.5% after 5 years (2).
Several mechanisms have been proposed to explain the return of the disease after surgery: the de novo recurrence of the endometriotic lesions (probably due to a retrograde menstruation) or the increasing of microscopic lesions persisting after surgery. The frequent relapse of the lesions on the same site of the previous surgical excision supports the last theory (4). Moreover, sometimes surgery is performed in a sub-optimal way, thus leaving on-site small lumps (5).
The adjuvant hormonal treatment is suggested by numerous evidences and by the guidelines from the European Society of Human Reproduction and Embryology (ESHRE) (6) in order to delay and prevent the recurrence of the disease after surgery and eradicate potential persistent spots of endometriosis. The medical treatment is reported to be more effective when started soon after surgery and when the duration of the treatment itself is long, since exogenous hormones are not healing but they exert a temporary suppressive action on a chronic and estrogen-dependent disease.
Short term treatment, inferior to six months, aims to symptoms control and to delay of the pain return, while a long-term treatment represents a long-term prevention of the disease, ideally avoiding his progression and the related symptoms, such as dysmenorrhea, chronic pelvic pain and deep dyspareunia (7).
However, compared to the short-term therapies, the long-term ones are burdened by more side effects, especially relevant for gonadotropin-releasing hormone agonist (GnRHa) and the danazol, and by costs (particularly for GnRHa and the aromatase inhibitors, AI). An add-back therapy is often administrated in association with GnRHa in order to improve the medications tolerability and the combination AI-GnRHa has been proposed too (8-12). On the other hand, the suspension of the suppressive therapy is associated to a return of the symptoms (13).
The aim of the present review is to assess the effectiveness of GnRHa after surgery for endometriosis in preventing the disease recurrence.
Materials and methods
Study selection and data extraction
We searched for studies evaluating the impact of GnRHa on the endometriosis recurrence. The searches were systematically conducted by two authors through PubMed, Embase, Medline, and Cochrane Library employing several key words: “endometriosis recurrence”, “gonadotropin-releasing hormone agonist”, “adjuvant therapy”, “pain recurrence”, “chronic pelvic pain” and “randomized controlled trial”. Moreover, a manual search was performed using the references cited in reviews and meta-analysis focused on the topic to identify further eligible studies.
We included only high quality randomized controlled trial (RCT) published in English and we collected studies in which the intervention was the GnRHa administration after surgery for endometriosis with the aim of assessing the recurrence rate.
The studies were excluded if the Jadad score was inferior to 3 (see Table S1), the study did not specify the recurrence rate after adjuvant therapy, the population did not undergo to a surgical treatment (some researchers performed only a diagnostic laparoscopy before hormonal therapy), the medical intervention was not postoperative and it was not specified which type of hormonal therapy was administered.
Assessment of study quality
Bias of each eligible study was assessed according to the Jadad scale (14) (Table S1), evaluating the randomization, the double blinding and the dropout and withdrawal reports of the trials. A score ranging between 3 and 5 was needed to design a trial as “high quality”.
Results
Literature search results
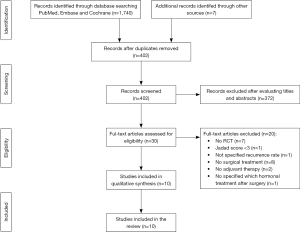
As showed in Figure 1, we identified 1747 studies, of which 402 were screened for eligibility. Finally, among the 30 eligible studies, 20 were excluded because they did not satisfy the inclusion criteria (15-34) and a total of 10 relevant RCT were included in the review (35-44).
Evaluation of included studies
We divided the included trials into three subgroups according to the recurrence parameter considered, as summarized in Tables 1-3. Three studies (35,41,42) reported an objective parameter of recurrence. They analyzed the rate of endometrioma relapse with an ultrasound exam or the endometriosis recurrence with a second line surgery (sometimes obtaining a histological exam) and/or with an increase of Ca125 serum levels. Four RCT (38,39,43,44) described a subjective recurrence parameter consisting in the severity of pain reported by the patients. Validate pain scales, such as verbal rating scale (VRS) or visual analogue scale (VAS), were employed for measuring the intensity of dysmenorrhea, chronic pelvic pain and dyspareunia. The remaining studies (36,37,40) considered both the subjective and objective recurrence.
Table 1
| Studies (all RCT) | Participants and intervention | Recurrence parameter | Follow up period and recurrence rate |
|---|---|---|---|
| Shaw |
Total n=48 | Endometrioma relapse (USG + histology-second-line surgery with excision of the cyst) | 6 months FU: |
| Goserelin group (3.6 mg subcutaneous injections monthly for 3 months) n=21 | Goserelin group 10% | ||
| Control group n=27 | Control group 15% | ||
| Acien |
Total n=52 | Recurrence of endometriosis (USG + histology-LPS second-look) + increased serum CA125 levels | 24–36 months FU: |
| Decapeptyl group (3.6 mg subcutaneous injections monthly for 6 months) n=26 | Decapeptyl group 26.9% | ||
| Control group n=26 | Control group 30.8% | ||
| Sesti |
Total n=259 | Endometrioma relapse (USG + second-look LPS) | 18 months FU: |
| Tryptorelin/Leuprorelin group (3.6 mg subcutaneous injections monthly for 6 months) n=65 | Triptorelin/Leuprorelin group 10.3% | ||
| Continuous OC group n=64 | OC group 15% | ||
| Placebo group n=65 | Placebo group 16.6% | ||
| Diet n=65 | Diet group 17.8% |
RCT, randomized controlled trial; USG, ultrasonography; FU, follow up; LPS, laparoscopy.
Table 2
| Studies (all RCT) | Participants and intervention | Recurrence parameter | Follow up period and recurrence rate |
|---|---|---|---|
| Hornstein |
Total n=201 | Patient-reported pain scores from baseline after surgery | 24 months FU: |
| Nafarelin group (200 pg intranasal twice daily for 6 months) n=49 | Nafarelin group 25% | ||
| Placebo group n=44 | Placebo group 47% (P=0.015) | ||
| Needed alternative therapy: | |||
| Nafarelin group 31% | |||
| Placebo group 57% (P<0.001) | |||
| Vercellini |
Total n=269 | VRS ≥5 (dysmenorrhoea, nonmenstrual pain, deep dyspareunia) | 12 months FU: |
| Goserelin group (3.6 mg subcutaneous injections monthly for 6 months) n=133 | Goserelin group 13.1% | ||
| Control group n=134 | Control group 21.4% (P=0.143) | ||
| 24 months FU: | |||
| Goserelin group 23.5 | |||
| Control group 36.5 (P=0.082) | |||
| Soysal |
Total n=80 | Biberoglu and Behrman scale for dysmenorrhoea, dyspareunia and pelvic pain Total Pelvic Symptom Score | 24 months FU: |
| Anastrozole (1 mg/day) + goserelin group (3.6 mg subcutaneous injections monthly for 6 months) n=40 | Anastrozole + goserelin group 45.3% | ||
| Placebo+ goserelin group (3.6 mg subcutaneous injections monthly for 6 months) n=40 | Placebo + goserelin group 89.6% | ||
| Granese |
Total n=78 | Pain recurrence (VAS for dysmenorrhea, dyspareunia and non-menstrual pelvic) + Endometriosis Health Profile questionnaire | 9 months FU: |
| OC group n=39 | OC group 10.8% | ||
| Leuprorelin/other GnRHa group (3.6 mg subcutaneous injections monthly for 6 months) n=39 | Leuprorelin/other GnRHa group 13.7% |
RCT, randomized controlled trial; FU, follow up; VRS, verbal rating scale; VAS, visual analogue scale
Table 3
| Studies (all RCT) | Participants and intervention | Recurrence parameter | Follow up period and recurrence rate |
|---|---|---|---|
| Busacca |
Total n=89 | Linear scale for dysmenorrhoea, pelvic pain, deep dyspareunia Multidimensional scale (limitation of daily activities, systemic symptoms, analgesics use) Biberoglu and Behrman scale (dyspareunia) | Median 19, range 6–36 months FU: |
| Leuprorelin group (3.6 mg subcutaneous injections monthly for 3 months) n=44 | Leuprolide group: Pain 23%; Objective disease 9% | ||
| Control group n=43 | Objective disease recurrence (USG) | Control group: Pain 29%; Objective disease 9% | |
| Loverro |
Total n=60 | Pain recurrence (VRS for dysmenorrhoea, pelvic pain, deep dyspareunia) + endometrioma relapse (USG) + increased serum CA125 levels | 60 months FU |
| Triptorelin group (3.6 mg subcutaneous injections monthly for 3 months) n=29 | Triptorelin group: Pain 44.8%; Endometrioma 21% | ||
| Placebo group n=25 | Placebo group: Pain 48%; Endometrioma 17.1% | ||
| Alborzi |
Total n=144 | Endometrioma relapse (USG) + pain recurrence (VAS for dysmenorrhea, dyspareunia and pelvic pain) | ≥12 months FU: |
| Letrozole group n=47 | Letrozole group 6.4% | ||
| Triptorelin group (3.6 mg subcutaneous injections monthly for 2 months) n=40 | Triptorelin group 5% | ||
| Control group n=57 | Control group 5.3% |
RCT, randomized controlled trial; FU, follow up; VRS, verbal rating scale; USG, ultrasonography; VAS, visual analogue scale.
The intervention of all the trials consisted by a short-term adjuvant therapy with a maximum treatment duration of 6 months.
Some of the included studies compared GnRHa to other hormonal therapies, of which two trials compared GnRHa to OC (38,41) and two others studied the impact of AI (36,43). Both the studies comprising OC group showed similar results concerning the recurrence rate compared to the GnRHa administration. One of them considered the endometrioma relapse (41) and the other one the symptoms recurrence (38). Concerning the AI effectiveness, the two mentioned studies described controversial results. Soysal et al. (43) reported a lower recurrence rate with the combination anastrozole-goserelin compared to the goserelin alone after 6 months of treatment, while Alborzi et al. (36) did not show any significant difference between triptorelin, letrozole and control group but the duration of the treatment was only 2 months for each group.
The remaining studies include in the present review showed conflicting results too. Three trials did not support the adjuvant GnRHa treatment (35,37,40) and other three reported a positive outcome on recurrence rate (39,42,44). Among the last cited, Vercellini et al. and Hornstein et al. (39,44) observed an increased pain-free interval and a delay of symptoms needing further treatment after GnRHa administration for 6 months after surgery, compared to no adjuvant treatment. Other researchers (42) reported their outcomes after laparoscopic aspiration of endometriomas, reporting a potential benefit of goserelin administration on diameter of the recurrent endometrioma. A smaller endometrioma size could offer an advantage for a second-line surgery.
Discussion
The use of the post-operative hormonal treatment after conservative or radical surgery for endometriosis is supported by numerous studies and by the ESHRE (6,10). Moreover, treatment duration seems to be crucial in the prevention of the disease (13,45).
The short-term treatment with GnRHa showed conflicting results. However, also OC short term treatment reported similar outcomes to GnRHa treatment concerning the recurrence rate (38,41). Muzii et al. (46) showed a low protective effect after adjuvant short term therapy with cyclic OC (although they reported a delay in symptoms recurrence compared to the control group), while other researchers (47-51) reported a beneficial impact of long term OC administration (for more than 6 months). Considering the available literature, it results reasonable to focus our attention on the treatment duration rather than on the kind of suppressive therapy used.
Considering that GnRHa are burdened by the well-known side effects related to hypoestrogenism, the reported studies did not employ them in a long-term period. When they are used for more than 6 months, an add back therapy should be considered. Although it is difficult to understand how much a short-term use of GnRHa influences the recurrence rate, more studies reported a longer pain free interval and a delay of recurrent endometriosis related symptoms.
Finally, we used the Jadad scale to assess potential bias of the studies. This quality scoring system includes the double-blinding among the “quality parameters”; however, most of the RCT are not double-blind (35-38,42,44) because it results difficult to conduct a double-blind study employing a medication with severe side effects.
Conclusions
Long term therapy plays a crucial role in the prevention of endometriosis related symptoms and endometriomas relapse after surgical treatment. However, the well-known side effects caused by GnRHa limit their use for a long period. The review reports conflicting results concerning the role of short-term therapy with GnRHa on recurrence rate, even if several studies describe a delay of recurrence of symptoms. Further researches are needed to clarify the impact of postoperative suppressive therapy also on deep infiltrating endometriosis (7).

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Medical Therapy in Endometriosis Treatment”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-31/coif). The series “Medical Therapy in Endometriosis Treatment” was commissioned by the editorial office without any funding or sponsorship. FB and SF served as the unpaid Guest Editors of the series. SF serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Oct 2018 to Sep 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update 2009;15:441-61. [Crossref] [PubMed]
- Vignali M, Bianchi S, Candiani M, et al. Surgical treatment of deep endometriosis and risk of recurrence. J Minim Invasive Gynecol 2005;12:508-13. [Crossref] [PubMed]
- Redwine DB, Wright JT. Laparoscopic treatment of complete obliteration of the cul-de-sac associated with endometriosis: long-term follow-up of en bloc resection. Fertil Steril 2001;76:358-65. [Crossref] [PubMed]
- Exacoustos C, Zupi E, Amadio A, et al. Recurrence of endometriomas after laparoscopic removal: sonographic and clinical follow-up and indication for second surgery. J Minim Invasive Gynecol 2006;13:281-8. [Crossref] [PubMed]
- Ferrero S, Petrera P, Remorgida V, et al. Endometriosis at 2nd surgery--residual or recurrent disease. Fertil Steril 2005;83:815-author reply 815-6. [Crossref] [PubMed]
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400-12. [Crossref] [PubMed]
- Ferrero S, Alessandri F, Racca A, et al. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril 2015;104:771-92. [Crossref] [PubMed]
- Tafi E, Leone Roberti Maggiore U, Alessandri F, et al. Advances in pharmacotherapy for treating endometriosis. Expert Opin Pharmacother 2015;16:2465-83. [Crossref] [PubMed]
- Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother 2018;19:1109-25. [Crossref] [PubMed]
- Ferrero S, Remorgida V, Venturini PL. Current pharmacotherapy for endometriosis. Expert Opin Pharmacother 2010;11:1123-34. [Crossref] [PubMed]
- Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol 2018;14:399-415. [Crossref] [PubMed]
- Bedaiwy MA, Allaire C, Alfaraj S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil Steril 2017;107:537-48. [Crossref] [PubMed]
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev 2004;CD003678 [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term follow-up. Fertil Steril 1993;59:511-5. [Crossref] [PubMed]
- Namnoum AB, Hickman TN, Goodman SB, et al. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril 1995;64:898-902. [Crossref] [PubMed]
- Donnez J, Nisolle M, Gillet N, et al. Large ovarian endometriomas. Hum Reprod 1996;11:641-6. [Crossref] [PubMed]
- Regidor PA, Regidor M, Kato K, et al. Long-term follow-up on the treatment of endometriosis with the GnRH-agonist buserelinacetate. Long-term follow-up data (up to 98 months) of 42 patients with endometriosis who were treated with GnRH-agonist buserelinacetate (Suprecur), were evaluated in respect of recurrence of pain symptoms and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 1997;73:153-60. [Crossref] [PubMed]
- Jee BC, Lee JY, Suh CS, et al. Impact of GnRH agonist treatment on recurrence of ovarian endometriomas after conservative laparoscopic surgery. Fertil Steril 2009;91:40-5. [Crossref] [PubMed]
- Park HJ, Koo YA, Yoon BK, et al. Postoperative long-term maintenance therapy with oral contraceptives after gonadotropin-releasing hormone analog treatment in women with ovarian endometrioma. J Minim Invasive Gynecol 2009;16:34-9. [Crossref] [PubMed]
- Mettler L, Ruprai R, Alkatout I. Impact of medical and surgical treatment of endometriosis on the cure of endometriosis and pain. Biomed Res Int 2014;2014:264653 [Crossref] [PubMed]
- Dlugi AM, Miller JD, Knittle J. Lupron depot (leuprolide acetate for depot suspension) in the treatment of endometriosis: a randomized, placebo-controlled, double-blind study. Lupron Study Group. Fertil Steril 1990;54:419-27. [Crossref] [PubMed]
- Fedele L, Bianchi S, Bocciolone L, et al. Buserelin acetate in the treatment of pelvic pain associated with minimal and mild endometriosis: a controlled study. Fertil Steril 1993;59:516-21. [Crossref] [PubMed]
- Parazzini F, Fedele L, Busacca M, et al. Postsurgical medical treatment of advanced endometriosis: results of a randomized clinical trial. Am J Obstet Gynecol 1994;171:1205-7. [Crossref] [PubMed]
- Bergqvist A, Bergh T, Hogstrom L, et al. Effects of triptorelin versus placebo on the symptoms of endometriosis. Fertil Steril 1998;69:702-8. [Crossref] [PubMed]
- Miller JD. Quantification of endometriosis-associated pain and quality of life during the stimulatory phase of gonadotropin-releasing hormone agonist therapy: a double-blind, randomized, placebo-controlled trial. Am J Obstet Gynecol 2000;182:1483-8. [Crossref] [PubMed]
- Kikuchi I, Takeuchi H, Kitade M, et al. Recurrence rate of endometriomas following a laparoscopic cystectomy. Acta Obstet Gynecol Scand 2006;85:1120-4. [Crossref] [PubMed]
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis. A multicenter double-blind comparative clinical trial. N Engl J Med 1988;318:485-9. [Crossref] [PubMed]
- Hornstein MD, Yuzpe AA, Burry KA, et al. Prospective randomized double-blind trial of 3 versus 6 months of nafarelin therapy for endometriosis associated pelvic pain. Fertil Steril 1995;63:955-62. [Crossref] [PubMed]
- Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol 2002;99:709-19. [PubMed]
- Koga K, Takemura Y, Osuga Y, et al. Recurrence of ovarian endometrioma after laparoscopic excision. Hum Reprod 2006;21:2171-4. [Crossref] [PubMed]
- Tsujioka H, Inoue Y, Emoto M, et al. The efficacy of preoperative hormonal therapy before laparoscopic cystectomy of ovarian endometriomas. J Obstet Gynaecol Res 2009;35:782-6. [Crossref] [PubMed]
- Alkatout I, Mettler L, Beteta C, et al. Combined surgical and hormone therapy for endometriosis is the most effective treatment: prospective, randomized, controlled trial. J Minim Invasive Gynecol 2013;20:473-81. [Crossref] [PubMed]
- Zhu S, Zhu Y, Liu Y, et al. Comparison of Outcomes of Different Postoperative Hormone Therapy in the Treatment of Ovarian Endometriosis: A Brief Report. Adv Ther 2018;35:857-63. [Crossref] [PubMed]
- Acien P, Quereda F, Campos A, et al. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: a randomized clinical trial. Fertil Steril 2002;78:705-11. [Crossref] [PubMed]
- Alborzi S, Hamedi B, Omidvar A, et al. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet 2011;284:105-10. [Crossref] [PubMed]
- Busacca M, Somigliana E, Bianchi S, et al. Post-operative GnRH analogue treatment after conservative surgery for symptomatic endometriosis stage III-IV: a randomized controlled trial. Hum Reprod 2001;16:2399-402. [Crossref] [PubMed]
- Granese R, Perino A, Calagna G, et al. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand 2015;94:637-45. [Crossref] [PubMed]
- Hornstein MD, Hemmings R, Yuzpe AA, et al. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril 1997;68:860-4. [Crossref] [PubMed]
- Loverro G, Carriero C, Rossi AC, et al. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III-IV endometriosis. Eur J Obstet Gynecol Reprod Biol 2008;136:194-8. [Crossref] [PubMed]
- Sesti F, Capozzolo T, Pietropolli A, et al. Recurrence rate of endometrioma after laparoscopic cystectomy: a comparative randomized trial between post-operative hormonal suppression treatment or dietary therapy vs. placebo. Eur J Obstet Gynecol Reprod Biol 2009;147:72-7. [Crossref] [PubMed]
- Shaw R, Garry R, McMillan L, et al. A prospective randomized open study comparing goserelin (Zoladex) plus surgery and surgery alone in the management of ovarian endometriomas. Gynaecol Endosc 2001;10:151-7. [Crossref]
- Soysal S, Soysal ME, Ozer S, et al. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod 2004;19:160-7. [Crossref] [PubMed]
- Vercellini P, Crosignani PG, Fadini R, et al. A gonadotrophin-releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. Br J Obstet Gynaecol 1999;106:672-7. [Crossref] [PubMed]
- Vercellini P, Somigliana E, Vigano P, et al. Post-operative endometriosis recurrence: a plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online 2010;21:259-65. [Crossref] [PubMed]
- Muzii L, Marana R, Caruana P, et al. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. Am J Obstet Gynecol 2000;183:588-92. [Crossref] [PubMed]
- Cucinella G, Granese R, Calagna G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Arch Gynecol Obstet 2013;288:821-7. [Crossref] [PubMed]
- Lee DY, Bae DS, Yoon BK, et al. Post-operative cyclic oral contraceptive use after gonadotrophin-releasing hormone agonist treatment effectively prevents endometrioma recurrence. Hum Reprod 2010;25:3050-4. [Crossref] [PubMed]
- Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010;93:52-6. [Crossref] [PubMed]
- Takamura M, Koga K, Osuga Y, et al. Post-operative oral contraceptive use reduces the risk of ovarian endometrioma recurrence after laparoscopic excision. Hum Reprod 2009;24:3042-8. [Crossref] [PubMed]
- Vercellini P, Somigliana E, Daguati R, et al. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol 2008;198:504.e1-5. [Crossref] [PubMed]
Cite this article as: D’Alessandro G, Barra F, Ferrero S. Use of GnRH analogue in the endometriosis recurrence after surgery. Gynecol Pelvic Med 2020;3:21.