Hormonal treatments for preventing recurrence of endometriomas
Introduction
Endometriosis is a chronic condition determined by the presence of ectopic endometrial glands and stroma. The disease affects approximately 10% of women of reproductive age and 35–50% of women with chronic pelvic pain and/or infertility (1-3). This condition is prevalent among the East Asian race, while African Americans are less frequently affected (4). Genetic, phenotypic, and lifestyle factors seem to influence the multifactorial etiology of endometriosis and endometriomas (5).
The ovaries are the most common implantation site of ectopic tissue and endometriotic cysts are one of the classic phenotype of the disorder (6). The cysts are more frequently unilateral, with a left-sided predisposition (7).
The etiopathogenesis of endometriosis is multifactorial and it cannot be supported by a single pathogenetic theory (8). Several hypotheses have been proposed, among which the in-situ theory and the transplantation theory: the first is based on endometriosis development by metaplasia of the germinal epithelium of the ovary or by embryological origin from mesonephric and Müllerian remnants (9-11). The transplantation theory, instead, is based on the concept that the endometriotic lesions may originate from ectopic endometrial tissue carried through the fallopian tubes during menstruation. In support of the theory of retrograde menstruation and the role of peritoneal fluid movements, the left predisposition of endometriomas may be explained by the anatomic barriers of the sigmoid colon that may delay the elimination of endometriotic tissue from the left hemipelvis and promote the ovarian implant (12-14).
Several mechanisms, both inherited and acquired, may influence in developing endometriosis. A large number of genes seem to have a combined action, coupled with epigenetic phenomena controlling the acquisition of immunologic, histological, and biological changes observed in patients affected by endometriosis (8). Some of these responsible genes regulate the expression of estrogen receptors and the cyclic estrogen and progesterone serum levels (15). A high estrogen concentration and an overexpression of estrogen receptor beta (ERβ), with an inversion of ERα to ERβ ratio in endometriotic tissue, seem to support estrogen-based ectopic cells survival; moreover, the progesterone resistance found in women affected plays a synergistic role in the maintenance of this dysregulated hormonal environment, considering the usual modulation in estrogen response by progesterone (16,17).
Transvaginal ultrasonography is a very sensitive and specific instrument for the endometrioma diagnosis. Unilocular cyst with a “ground glass” homogeneity, low levels of echogenicity, and poor vascularization are typical ultrasound characteristics of endometriomas (18,19).
Surgical and/or medical therapies for endometriomas aim to control symptoms (in particular, chronic pelvic pain and dysmenorrhea) and prevent cyst growth; medical therapy may be employed to reduce recurrence rate after surgery. Unfortunately, the reported postoperative recurrence rate of endometriomas is high, ranging from 30% to 40%, representing a source of frustration for the gynecologist in the management of this chronic condition (6,20-25).
There are no medical therapies that effectively treat existing endometriomas. Therefore, tertiary prevention, consisting in a pharmacological adjuvant treatment, takes place after surgical excision in order to prevent the disease recurrence and to minimize the risk of damage to the ovarian reserve, especially in women desiring to conceive in the future (26-29). Moreover, medical therapy should be started shortly after cystectomy, considering the speed of disease recurrence (30).
It is reported that long-term suppressive hormonal treatment has a crucial role in preventing the recurrence of ovarian endometriomas (31). Several hormonal alternatives have been proposed and are employed in the clinical practice, such as oral contraceptives (OC), progestins (PG), GnRH analogs (GnRHa), danazol, aromatase inhibitors (AI), GnRH antagonists, selective estrogen receptor modulators (SERMs) and selective progesterone receptor modulators (SPRMs) (32,33). In parallel or in addition to hormonal therapy, alternative phytotherapeutic options, such as medicinal plants, phytochemicals, and multi-component herbal preparations, have been investigated, obtaining promising results. In fact, some of these compounds appear to influence epigenetic factors, apoptosis, and cell survival as well as angiogenetic processes and oxidative stress. Moreover, specific agents have a role in the estrogen modulation (34-36).
However, hormonal treatment remains the most employed option for treating pain related to endometriosis and therefore the use of phototherapies should be limited to the scientific research setting. The suppression of ovulation and the reduction in retrograde menstruation, two factors that have a crucial role in the development of the disease, are potential mechanisms by which OC and PG act. These drugs represent the most common medical therapy for endometriomas due to the low cost and the favorable safety profile (37-39). These medications should be assumed regularly as long-term regimens in case of the patients have not desire of conception. If administrated continuously, these compounds can induce amenorrhea, totally resolving dysmenorrhea and avoiding retrograde menstruation (31,40).
The levonorgestrel-releasing intrauterine system (LNG-IUS) has been employed in this context. This device does not suppress ovulation (except for a few months after insertion); however, it reduces or abolishes the menstrual flow (41,42).
GnRHa represent another widely used choice. They have a beneficial impact on reducing pain and recurrence of endometriosis after surgery. However, they are more expensive and poorly tolerated due to the side effects related to the profound hypoestrogenism, such as hot flushes, urogenital atrophy, loss of libido, deterioration in the lipid profile, depression, and bone loss. Therefore, GnRHa are commonly administrated in combination with “add-back” therapy (consisting of norethisterone acetate, low-dose estrogen-progestin replacement, OC, and bisphosphonates) (33,43).
There are still few available data about AI, GnRH antagonists, and danazol regarding their impact in the context of endometrioma therapy (44,45).
The present review aims to evaluate the efficacy of adjuvant hormonal treatments after surgery for preventing endometrioma recurrence.
Materials and methods
For the current review, we systematically searched PubMed, Embase and Cochrane Library for relevant studies on hormonal treatments for preventing endometrioma recurrence after surgery, using the following key words: “endometrioma recurrence”, “hormonal treatments”, “adjuvant therapy”, “endometrioma excision”, “GnRH agonist”, “oral contraceptives”, “progestins”, “levonorgestrel-releasing intrauterine system”, “danazol”, “aromatase inhibitors”. The searches were conducted by two authors, first by reading the title and the abstract and then by reading the full articles.
We included studies that met the following criteria: randomized controlled trial (RCT) or cohort studies with control or placebo group, a study group undergoing a surgical procedure for endometrioma and an adjuvant hormonal therapy, studies reporting endometrioma recurrence rate among outcomes and studies published in English. We excluded case reports, review or meta-analysis, studies published as abstract, study groups consisting of patients with endometriosis without specifying if they had an ovarian endometrioma and studies reporting only pregnancy outcomes among results.
A validated scale (Newcastle-Ottawa scale) was used for quality assessment of the included studies (Table S1).
Results
Study selection
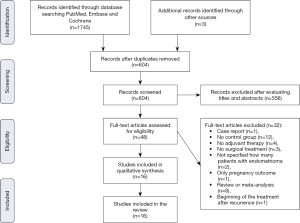
The systematic search of Medline and other sources identified 1,748 studies and 48 articles were assessed for eligibility. We excluded one study as it was a case report (45), 12 studies in which there was no control group (24,46-56), four studies in which the hormonal therapy was not administered postoperatively (22,57-59), three studies because patients did not undergo surgical treatment of endometrioma (44,60,61), two studies as they did not clarify if patients with endometriosis had an ovarian endometriotic cyst (62,63), one study which did not report the endometrioma relapse rate (focusing only on the pregnancy outcome) (64), eight review or meta-analysis (31,39,43,65-69) and one study because the hormonal treatment began after the endometrioma recurrence (70). Finally, data evaluating the impact of postoperative hormonal treatment on prevention of endometrioma were extracted from 16 articles, of which eight were RCT (71-78) and eight were cohort studies (6,25,79-84). The process for identifying the relevant studies is shown in Figure 1.
Description of the studies and summary of the outcomes
The main details and the design of the studies included in the review are summarized in Table 1. Six studies evaluated the effect of OC on the recurrence of endometrioma after laparoscopic excision of the cyst (25,71,76,80-82), four studies reported the effect of adjuvant GnRHa treatment (72-74,79), two others compared GnRHa administration to PG use (6,84), one compared GnRHa to OC (75) and another to AI (77), one investigated the efficacy of PG (83) and another one showed the effect of LNG-IUS (78). All studies included a control or placebo group.
Table 1
| Study | Type of study | No. of patients [age] | Surgical treatment | Adiuvant medical treatment [No.] | Treatment period (months) | Follow up (months) | Recurrence parameter (method of measurement) |
|---|---|---|---|---|---|---|---|
| Muzii |
RCT | 70 [20–35] | LPS excision of endometriomas | Low-dose cyclic OC [33] | 6 | Mean 22 (range, 12–48) | Endometrioma relapse (US) or pain (≥4 on VAS) |
| Controls [35] | |||||||
| Shaw |
Multi-centre RCT | 48 [18-50] | LPS aspiration of endometrioma | GnRHa (Goserelin) [21] | 3 | 6 | Endometrioma relapse (US+ histology - second-line surgery with excision of the cyst) |
| Controls [27] | |||||||
| Acien |
RCT | 52 [24-37] | LPT excision of endometrioma | GnRHa (Decapeptyl) [26] | 6 | 24–36 | Recurrence of endometriosis (US+ histology- LPS second-look) + increased serum CA125 levels |
| Controls [26] | |||||||
| Liu |
Retrospective cohort study | 710 [reproductive age] | LPS or LPT (conservative/semiradical) | GnRHa [224] | Not specified | Mean 22.4 (range, 11.8–38.8) | Recurrence of endometrioma (TV-US)± symptoms (pain recurred after 3 months from surgery, |
| PG [95] | with the severity score equal to or higher than that | ||||||
| Others [23] | before the surgery) | ||||||
| Controls [368] | |||||||
| Loverro |
RCT | 60 [24–33] | LPS excision in case of endometriomas | GnRHa (Triptorelin) [29] | 3 | 60 | Pain recurrence (VRS) + endometrioma relapse (TV-US)+ increased serum CA125 levels |
| (65% with endometrioma) | Placebo [25] | ||||||
| Vercellini |
Prospective cohort study | 277 [<40] | LPS excision of endometriomas | Cyclic OC [102] | For the entire follow up period | Median 28 (range, 17–45) | Endometrioma relapse (TV-US + histology - second-line surgery) |
| Controls [46] | |||||||
| Sesti |
RCT | 259 [>40] | LPS excision of endometriomas | GnRHa (Tryptorelin or Leuprorelin) [65] | 6 | 18 | Endometrioma relapse (tV-US + second-look LPS) |
| Continuous OC [64] | |||||||
| Placebo [65] | |||||||
| Diet [65] | |||||||
| Jee |
Retrospective cohort study | 109 [premenopausal age] | LPS excision of endometriomas | GnRHa [72] | 3, 4 or 6 | Mean 20.1 (range, 6–43) | Endometrioma relapse (TV-US) |
| Controls [37] | |||||||
| Takamura |
Retrospective cohort study | 87 [<40] | LPS excision of endometriomas | Cyclic OC (always users]) [34] | For the entire follow up period | 24 | Endometrioma relapse (TV-US) |
| Discontinued OC (ever users) [14] | |||||||
| Controls [39] | |||||||
| Alborzi |
RCT | 144 [reproductive age] | LPS excision of endometriomas + adhesionolysis | AI (letrozole) [47] | 2 | ≥12 | Endometrioma relapse (TV-US)+ symptoms (VAS) |
| GnRHa (Triptorelin) [40] | |||||||
| Controls [57] | |||||||
| Seracchioli |
RCT | 239 [20-40] | LPS excision of endometriomas | Cyclic OC [75] | For the entire follow up period. | 24 | Endometrioma relapse (TV-US) |
| Continuous OC [73] | |||||||
| Controls (non users) [63] | |||||||
| Lee |
Retrospective cohort study | 362 [reproductive age] | LPS excision of endometriomas | Cyclic OC always users (after GnRHa treatment]) [139]+ discontinued OC ever users (after GnRHa treatment) [36] | For the entire follow up period | Median 35 (range, 12–114) | Endometrioma relapse (TV-US) |
| Controls (only GnRHa treatment) [187] | |||||||
| Cucinella |
Multi-centre prospective cohort study | 168 [18–40] | LPS excision of endometriomas | Cyclic OC [126] | For the entire follow up period | 24 | Endometrioma relapse (TV-US) |
| Controls [38] | |||||||
| Chen |
RCT | 80 [20–43] | LPS excision of endometriomas | LNG-IUS [40] | For the entire follow up period. | 30 | Endometrioma relapse (TV-US) |
| Controls [40] | + serum CA125 levels +dysmenorrhea and noncyclic pelvic pain (VAS) | ||||||
| Yamanaka |
Retrospective cohort study | 126 [28–42] | LPS excision of endometriomas + USLs resection | PG (DNG) [59] | Mean 31±17.6 | Mean 32±16.3 | Endometrioma relapse (TV-US) or pain (VAS returning to preoperative levels) or endometriosis-related symptoms after |
| Controls [67] | operation (VAS score ≥4) | ||||||
| Zhu |
Retrospective cohort study | 399 [20–38] | LPS excision of endometriomas | PG (norethindrone 1.2 mg/day) | 24 | 48 | Endometrioma relapse (not specified) |
| PG (norethindrone 5.0 mg/day) [236] | |||||||
| GnRHa [96] | |||||||
| Controls [67] |
RCT, randomized controlled trial; LPS, laparoscopy; OC, oral contraceptives; US, ultrasonography; VAS, visual analogue scale; GnRHa, gonadotrophin-releasing hormone agonists; LPT, laparotomic; PG, progestins; TV-US, transvaginal ultrasonography; VRS, verbal rating scale; AI, aromatase inhibitor; LNG-IUS, levonorgestrel-releasing intrauterine system; USLs, uterosacral ligaments; DNG, dienogest.
The duration of the treatment coincided with the follow-up period in only six studies (25,76,80-83), while it was shorter in the other ones.
Almost all the included studies considered the endometrioma recurrence by an ultrasonographic exam [the only one which did not clarify the method of endometrioma relapse assessment was the study by Zhu et al. (84)] and some of them considered also the symptomatology (6,71,74,78,83) or the increased in serum CA125 level (73,74,78) as a sign of disease recurrence. Few studies reported also a surgical and histological confirmation of the endometriotic recurrent cyst (25,72,73,75).
Concerning the recurrence rate (see Table 2), seven studies reported an advantage on the prevention of the endometrioma relapse by postoperative hormonal treatment (25,72,76,80-83). Among these studies, the RCT by Shaw et al. (72) was poorly comparable with the other studies (Table S1) because the therapy was administrated after surgical aspiration (instead of an excision) of the endometriotic cyst. The authors reported that the use of goserelin for only three months may reduce the endometrioma size offering a potential advantage for a second-line surgery. Seracchioli et al. (76) found that the mean cyst recurrent diameter increased every 6 months of follow-up especially in the control group (non-users OC) (0.48±0.3 cm) compared to cyclic OC users (0.31±0.18 cm) and continuous OC users (0.25±0.09 cm).
Table 2
| Study | Recurrence rate | P value |
|---|---|---|
| Muzii |
Low-dose cyclic OC: 6.1% (endometrioma)—9.1% (pain) | NS |
| Controls: 2.9% (endometrioma)—17.1% (pain) | ||
| Shaw |
GnRHa: 10% | Not specified |
| Controls: 15% | ||
| Acien |
GnRHa: 26.9% | Not specified |
| Controls: 30.8% | ||
| Liu |
GnRHa: 43.8% | NS |
| PG: 14.6% | ||
| Others: 4.2% | ||
| Controls: 37.5% | ||
| Loverro |
GnRHa 44.8% (pain)—21% (endometrioma) | NS |
| Controls: 48% (pain)—17.1% (endometrioma) | ||
| Vercellini |
Cyclic OC: 9% | <.001 |
| Controls: 56% | ||
| Sesti |
GnRHa: 10.3% | NS |
| OC: 15% | ||
| Placebo: 16.6% | ||
| Diet: 17.8% | ||
| Jee |
GnRHa: 17.9% (3 months)—28.6% (4 months)—4.3% (6months) | NS |
| Controls: 16.2% | ||
| Takamura |
Cyclic OC (always users): 2.9% | 0.001 |
| Cyclic OC (ever users): 14.3% | ||
| Controls: 43.5% | ||
| Alborzi |
AI: 6.4% | NS |
| GnRHa: 5% | ||
| Controls: 5.3% | ||
| Seracchioli |
Cyclic OC: 14.7% | 0.003 |
| Continuous OC: 8.2% | ||
| Controls (non users): 29% | ||
| Lee |
Cyclic OC (always users+ ever users): 7.4% | 0.001 |
| Controls: 28.9°% | ||
| Cucinella |
Cyclic OC: 8% | 0.001 |
| Controls: 39% | ||
| Chen |
LNG-IUS: | NS endometrioma |
| 25% (endometrioma) | <0.001 dysmenorrhea | |
| 38.7±25.9 mean reduction dysmenorrhea (VAS) | 0.014 noncyclic pelvic pain | |
| 30.1±14.7 mean reduction noncyclic pelvic pain (VAS) | 0.001 Ca125 | |
| –15.6 median reduction Ca125 | ||
| Controls: | ||
| 37.5% (endometrioma) | ||
| 60.8±25.5 mean reduction dysmenorrhea (VAS) | ||
| 39.1±10.9 mean reduction noncyclic pelvic pain (VAS) | ||
| –32.1 median reduction Ca125 | ||
| Yamanaka |
PG: 5% (endometrioma)—0% (pain)—6.7% (symptoms) | 0.0002 endometrioma |
| Controls: 31.3% (endometrioma)—11.9% (pain)—43.2% (symptoms) | 0.0061 pain | |
| 0.0001 symptoms | ||
| Zhu |
After 24 months (at the end of the treatment): | >0.05 |
| PG (lower dose): 8% | ||
| PG (higher dose): 9% | ||
| GnRHa: 13% | ||
| Controls: 39% | ||
| After 48 months (compared with recurrence rate during the treatment): | ||
| PG (lower dose): 19% | ||
| PG (higher dose): 19% | ||
| GnRHa: 24% | ||
| Controls: 13% |
OC, oral contraceptives; NS, not significant; GnRHa, gonadotrophin-releasing hormone agonists; PG, progestins; AI, aromatase inhibitor; LNG-IUS, levonorgestrel-releasing intrauterine system; VAS, visual analogue scale
Cucinella et al. (82) compared the control group (women who refused the adjuvant therapy) to the cyclic OC users divided into three subgroups according to different progestin types (monophasic desogestrel, monophasic gestodene, and biphasic dienogest). They observed 74.7% cumulative probability of cyst recurrence among non-users, while a very lower risk among the other three subgroups (respectively 26.5%, 31.8%, and 20.5%). The mean recurrent endometrioma diameter showed a better trend among users (respectively 1.9, 1.8, 1.3 cm) compared to non-users (3.1 cm; P=0.0001).
Other researchers (83) operated 126 patients with endometriotic cyst and deep infiltrating endometriosis testing the effect of dienogest (DNG) on recurrent pain and endometriomas. They observed a significant decrease of endometriosis-related pain and endometriomas after the therapy compared to the control group. However, among other endometriosis-related symptoms (dysmenorrhea, non-menstrual pelvic pain, dyspareunia, and dyschezia), they did not find any statistically significant difference between DNG users and non-users, except for dysmenorrhea (P<0.001).
Finally, five studies did not report any advantage on the prevention of the endometrioma relapse with postoperative hormonal treatment (71,73-75,77). The other studies included in the review showed inconsistent results or different conclusions. The study with the largest population (6) (710 women) was inconclusive concerning the beneficial effect of postoperative hormonal treatment (P value not statistically significant); whereas it reported other factors to be more associated with the recurrence (previous medication use, previous surgery, total Revised American Fertility Society score and younger age at surgery).
Jee et al. (79) reported different results. They compared three different durations of GnRHa treatment (3, 4 and 6 months) to a control group, observing a low recurrence rate only after 6 months of treatment, while shorter therapy or no therapy had not a beneficial impact on the recurrence rate after conservative laparoscopic surgery for ovarian endometriomas.
Discussion
The five studies (71,73-75,77) reporting no beneficial use of adjuvant treatment in terms of recurrence rate for endometriomas were based on a short-term usage of hormonal therapy (maximum 6 months) and the ultrasound assessment of the cyst relapse was performed several months after the end of the treatment. Only one study (78) reported no beneficial effect despite an equivalent period of treatment and follow up (30 months); this study is the only one included in the present review evaluating LNG-IUS as adjuvant treatment. However, the results are inconsistent with other studies concerning the postoperative use of LNG-IUS (not included because of the absence of a control group). Taneja et al. (54) compared LNG-IUS and danazol reporting that the first one was more effective in relieving pain (65.2% vs. 38.0%, P<0.05) and preventing the recurrence. In contrast, Cho et al. (52) found no significant difference between postoperative LNG-IUS use and cyclic OC in preventing endometrioma recurrence (P=0.461). Other researchers (48) compared depot medroxyprogesterone acetate with LNG-IUS, not observing a significant difference in the recurrence rate of endometriomas between both groups. Moreover, these authors reported better compliance in the LNG-IUS group with a beneficial effect on symptoms control and prevention of recurrence.
All the remaining studies reporting a treatment period lasting almost the entire follow-up observed an advantage by the postoperative hormonal therapy (25,76,80-83).
The retrospective cohort study by Zhu et al. (84) is the only study that reported the endometrioma recurrence rate both after 12 months (at the end of the medical treatment) and after 24 months (12 months after the end of the medical treatment). After the first year, the control group showed more recurrence rate, whereas after the treatment suspension the medical beneficial effect seemed to be less relevant.
Conclusions
Several hormonal compounds, such as OC, PG, and GnRHa, have proven to be beneficial on endometrioma recurrence even if some studies reported conflicting results. It is unclear if one postoperative hormonal treatment is superior to the others in the prevention of endometrioma recurrence. However, the duration of adjuvant therapy seems to have a crucial role in this field. There are still few studies concerning the role of danazol, AI, SERMs, and SPRMS in the postoperative period and other evidences are needed to clarify which hormonal treatment may represent the best choice.

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Medical Therapy in Endometriosis Treatment”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-23/coif). The series “Medical Therapy in Endometriosis Treatment” was commissioned by the editorial office without any funding or sponsorship. FB served as an unpaid Guest Editor of the series. SF served as an unpaid Guest Editor of the series and serves as an editorial board member of Gynecology and Pelvic Medicine from Oct 2018 to Sept 2020. The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789-99. [Crossref] [PubMed]
- Meuleman C, Vandenabeele B, Fieuws S, et al. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril 2009;92:68-74. [Crossref] [PubMed]
- Ferrero S, Arena E, Morando A, et al. Prevalence of newly diagnosed endometriosis in women attending the general practitioner. Int J Gynaecol Obstet 2010;110:203-7. [Crossref] [PubMed]
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30:1-19. vii. [Crossref] [PubMed]
- Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci 2008;1127:92-100. [Crossref] [PubMed]
- Liu X, Yuan L, Shen F, et al. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol 2007;109:1411-20. [Crossref] [PubMed]
- Ferrero S, Ragni N, Fulcheri E. Lateral distribution of benign ovarian cysts. Int J Gynaecol Obstet 2005;89:150-1. [Crossref] [PubMed]
- Laganà AS, Garzon S, Götte M, et al. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int J Mol Sci 2019;20:5615. [Crossref] [PubMed]
- Lauchlan SC. The secondary Mullerian system. Obstet Gynecol Surv 1972;27:133-46. [Crossref] [PubMed]
- Batt RE, Yeh J. Mullerianosis: four developmental (embryonic) mullerian diseases. Reprod Sci 2013;20:1030-7. [Crossref] [PubMed]
- van der Linden PJ. Theories on the pathogenesis of endometriosis. Hum Reprod 1996;11:53-65. [Crossref] [PubMed]
- Ulukus M, Yeniel AO, Ergenoglu AM, et al. Right endometrioma is related with more extensive obliteration of the Douglas pouch. Arch Gynecol Obstet 2012;285:1483-6. [Crossref] [PubMed]
- Bricou A, Batt RE, Chapron C. Peritoneal fluid flow influences anatomical distribution of endometriotic lesions: why Sampson seems to be right. Eur J Obstet Gynecol Reprod Biol 2008;138:127-34. [Crossref] [PubMed]
- Vercellini P, Pisacreta A, Vicentini S, et al. Lateral distribution of nonendometriotic benign ovarian cysts. BJOG 2000;107:556-8. [Crossref] [PubMed]
- Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 2008;55:795-810. [Crossref] [PubMed]
- McKinnon B, Mueller M, Montgomery G. Progesterone Resistance in Endometriosis: an Acquired Property? Trends Endocrinol Metab 2018;29:535-48. [Crossref] [PubMed]
- Bulun SE, Monsavais D, Pavone ME, et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med 2012;30:39-45. [Crossref] [PubMed]
- Endometriosis Treatment Italian Club. Ovarian endometrioma: what the patient needs. J Minim Invasive Gynecol 2014;21:505-16. [Crossref] [PubMed]
- Guerriero S, Ajossa S, Mais V, et al. The diagnosis of endometriomas using colour Doppler energy imaging. Hum Reprod 1998;13:1691-5. [Crossref] [PubMed]
- Kim ML, Kim JM, Seong SJ, et al. Recurrence of ovarian endometrioma after second-line, conservative, laparoscopic cyst enucleation. Am J Obstet Gynecol 2014;210:216.e1-6. [Crossref] [PubMed]
- Vercellini P, Somigliana E, Vigano P, et al. Endometriosis: current therapies and new pharmacological developments. Drugs 2009;69:649-75. [Crossref] [PubMed]
- Kikuchi I, Takeuchi H, Kitade M, et al. Recurrence rate of endometriomas following a laparoscopic cystectomy. Acta Obstet Gynecol Scand 2006;85:1120-4. [Crossref] [PubMed]
- Busacca M, Chiaffarino F, Candiani M, et al. Determinants of long-term clinically detected recurrence rates of deep, ovarian, and pelvic endometriosis. Am J Obstet Gynecol 2006;195:426-32. [Crossref] [PubMed]
- Koga K, Takemura Y, Osuga Y, et al. Recurrence of ovarian endometrioma after laparoscopic excision. Hum Reprod 2006;21:2171-4. [Crossref] [PubMed]
- Vercellini P, Somigliana E, Daguati R, et al. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol 2008;198:504.e1-5. [Crossref] [PubMed]
- Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother 2018;19:1109-25. [Crossref] [PubMed]
- Leone Roberti Maggiore U, Gupta JK, Ferrero S. Treatment of endometrioma for improving fertility. Eur J Obstet Gynecol Reprod Biol 2017;209:81-5. [Crossref] [PubMed]
- Tafi E, Leone Roberti Maggiore U, Alessandri F, et al. Advances in pharmacotherapy for treating endometriosis. Expert Opin Pharmacother 2015;16:2465-83. [Crossref] [PubMed]
- Ferrero S, Alessandri F, Racca A, et al. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril 2015;104:771-92. [Crossref] [PubMed]
- Falcone T, Flyckt R. Clinical Management of Endometriosis. Obstet Gynecol 2018;131:557-71. [Crossref] [PubMed]
- Somigliana E, Vercellini P, Vigano P, et al. Postoperative medical therapy after surgical treatment of endometriosis: from adjuvant therapy to tertiary prevention. J Minim Invasive Gynecol 2014;21:328-34. [Crossref] [PubMed]
- Barra F, Grandi G, Tantari M, et al. A comprehensive review of hormonal and biological therapies for endometriosis: latest developments. Expert Opin Biol Ther 2019;19:343-60. [Crossref] [PubMed]
- Platteeuw L, D'Hooghe T. Novel agents for the medical treatment of endometriosis. Curr Opin Obstet Gynecol 2014;26:243-52. [Crossref] [PubMed]
- Scutiero G, Iannone P, Bernardi G, et al. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid Med Cell Longev 2017;2017:7265238 [Crossref] [PubMed]
- Taniguchi F, Kaponis A, Izawa M, et al. Apoptosis and endometriosis. Front Biosci (Elite Ed) 2011;3:648-62. [Crossref] [PubMed]
- Della Corte L, Noventa M, Ciebiera M, et al. Phytotherapy in endometriosis: an up-to-date review. J Complement Integr Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Leyland N, Casper R, Laberge P, et al. Endometriosis: diagnosis and management. J Obstet Gynaecol Can 2010;32:S1-32. [Crossref] [PubMed]
- . Practice bulletin no. 114: management of endometriosis. Obstet Gynecol 2010;116:223-36. [Crossref] [PubMed]
- Vercellini P, DE, Matteis S, Somigliana E, et al. Long-term adjuvant therapy for the prevention of postoperative endometrioma recurrence: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2013;92:8-16. [Crossref] [PubMed]
- Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod 2014;29:400-12. [Crossref] [PubMed]
- Barra F, Scala C, Ferrero S. Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol 2018;14:399-415. [Crossref] [PubMed]
- Muzii L. Medicated intrauterine systems for treatment of endometriosis-associated pain. J Minim Invasive Gynecol 2006;13:535-8. [Crossref] [PubMed]
- Rehmer JM, Flyckt RL, Goodman LR, et al. Management of Endometriomas. Obstet Gynecol Surv 2019;74:232-40. [Crossref] [PubMed]
- Agarwal SK, Foster WG. Reduction in Endometrioma Size with Three Months of Aromatase Inhibition and Progestin Add-Back. Biomed Res Int 2015;2015:878517 [Crossref] [PubMed]
- Lall Seal S, Kamilya G, Mukherji J, et al. Aromatase inhibitors in recurrent ovarian endometriomas: report of five cases with literature review. Fertil Steril 2011;95:291.e15-8. [PubMed]
- Donnez J, Nisolle M, Gillet N, et al. Large ovarian endometriomas. Hum Reprod 1996;11:641-6. [Crossref] [PubMed]
- Park HJ, Koo YA, Yoon BK, et al. Postoperative long-term maintenance therapy with oral contraceptives after gonadotropin-releasing hormone analog treatment in women with ovarian endometrioma. J Minim Invasive Gynecol 2009;16:34-9. [Crossref] [PubMed]
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol 2010;50:273-9. [Crossref] [PubMed]
- Muzii L, Maneschi F, Marana R, et al. Oral estroprogestins after laparoscopic surgery to excise endometriomas: continuous or cyclic administration? Results of a multicenter randomized study. J Minim Invasive Gynecol 2011;18:173-8. [Crossref] [PubMed]
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Womens Health 2012;4:149-54. [Crossref] [PubMed]
- Vlahos N, Vlachos A, Triantafyllidou O, et al. Continuous versus cyclic use of oral contraceptives after surgery for symptomatic endometriosis: a prospective cohort study. Fertil Steril 2013;100:1337-42. [Crossref] [PubMed]
- Cho S, Jung JA, Lee Y, et al. Postoperative levonorgestrel-releasing intrauterine system versus oral contraceptives after gonadotropin-releasing hormone agonist treatment for preventing endometrioma recurrence. Acta Obstet Gynecol Scand 2014;93:38-44. [Crossref] [PubMed]
- Kim ML, Cho YJ, Kim MK, et al. The efficacy of long-term maintenance therapy with a levonorgestrel-releasing intrauterine system for prevention of ovarian endometrioma recurrence. Int J Gynaecol Obstet 2016;134:256-9. [Crossref] [PubMed]
- Taneja A, Kaur S, Soni RK, et al. Evaluating the Efficacy of Levonorgestrel Intrauterine System and Danazol for Relief of Postoperative Pain in Endometriosis. J Clin Diagn Res 2017;11:QC10-2. [PubMed]
- Seo JW, Lee DY, Kim SE, et al. Comparison of long-term use of combined oral contraceptive after gonadotropin-releasing hormone agonist plus add-back therapy versus dienogest to prevent recurrence of ovarian endometrioma after surgery. Eur J Obstet Gynecol Reprod Biol 2019;236:53-7. [Crossref] [PubMed]
- Angioni S, Pontis A, Malune ME, et al. Is dienogest the best medical treatment for ovarian endometriomas? Results of a multicentric case control study. Gynecol Endocrinol 2020;36:84-6. [Crossref] [PubMed]
- Kavoussi SK, Odenwald KC, As-Sanie S, et al. Incidence of ovarian endometrioma among women with peritoneal endometriosis with and without a history of hormonal contraceptive use. Eur J Obstet Gynecol Reprod Biol 2017;215:220-3. [Crossref] [PubMed]
- Tsujioka H, Inoue Y, Emoto M, et al. The efficacy of preoperative hormonal therapy before laparoscopic cystectomy of ovarian endometriomas. J Obstet Gynaecol Res 2009;35:782-6. [Crossref] [PubMed]
- Westhoff C, Britton JA, Gammon MD, et al. Oral contraceptive and benign ovarian tumors. Am J Epidemiol 2000;152:242-6. [Crossref] [PubMed]
- Del Forno S, Mabrouk M, Arena A, et al. Dienogest or Norethindrone acetate for the treatment of ovarian endometriomas: Can we avoid surgery? Eur J Obstet Gynecol Reprod Biol 2019;238:120-4. [Crossref] [PubMed]
- Harada T, Momoeda M, Taketani Y, et al. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril 2008;90:1583-8. [Crossref] [PubMed]
- Morelli M, Sacchinelli A, Venturella R, et al. Postoperative administration of dienogest plus estradiol valerate versus levonorgestrel-releasing intrauterine device for prevention of pain relapse and disease recurrence in endometriosis patients. J Obstet Gynaecol Res 2013;39:985-90. [Crossref] [PubMed]
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, et al. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-related pain: a randomized controlled trial. Obstet Gynecol 2012;119:519-26. [Crossref] [PubMed]
- Kolanska K, Cohen J, Bendifallah S, et al. Pregnancy outcomes after controlled ovarian hyperstimulation in women with endometriosis-associated infertility: GnRH-agonist versus GnRH-antagonist. J Gynecol Obstet Hum Reprod 2017;46:681-6. [Crossref] [PubMed]
- Song SY, Park M, Lee GW, et al. Efficacy of levonorgestrel releasing intrauterine system as a postoperative maintenance therapy of endometriosis: A meta-analysis. Eur J Obstet Gynecol Reprod Biol 2018;231:85-92. [Crossref] [PubMed]
- Zheng Q, Mao H, Xu Y, et al. Can postoperative GnRH agonist treatment prevent endometriosis recurrence? A meta-analysis. Arch Gynecol Obstet 2016;294:201-7. [Crossref] [PubMed]
- Seracchioli R, Mabrouk M, Manuzzi L, et al. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum Reprod 2009;24:2729-35. [Crossref] [PubMed]
- Vercellini P, Somigliana E, Vigano P, et al. Endometriosis: current and future medical therapies. Best Pract Res Clin Obstet Gynaecol 2008;22:275-306. [Crossref] [PubMed]
- Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev 2004;2004:CD003678 [PubMed]
- Koshiba A, Mori T, Okimura H, et al. Dienogest therapy during the early stages of recurrence of endometrioma might be an alternative therapeutic option to avoid repeat surgeries. J Obstet Gynaecol Res 2018;44:1970-6. [PubMed]
- Muzii L, Marana R, Caruana P, et al. Postoperative administration of monophasic combined oral contraceptives after laparoscopic treatment of ovarian endometriomas: a prospective, randomized trial. Am J Obstet Gynecol 2000;183:588-92. [Crossref] [PubMed]
- Shaw R, Garry R, McMillan L, et al. A prospective randomized open study comparing goserelin (Zoladex) plus surgery and surgery alone in the management of ovarian endometriomas. Gynaecol Endosc 2001;10:151-7. [Crossref]
- Acién P, Quereda F, Campos A, et al. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: a randomized clinical trial. Fertil Steril 2002;78:705-11. [Crossref] [PubMed]
- Loverro G, Carriero C, Rossi AC, et al. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III-IV endometriosis. Eur J Obstet Gynecol Reprod Biol 2008;136:194-8. [Crossref] [PubMed]
- Sesti F, Capozzolo T, Pietropolli A, et al. Recurrence rate of endometrioma after laparoscopic cystectomy: a comparative randomized trial between post-operative hormonal suppression treatment or dietary therapy vs. placebo. Eur J Obstet Gynecol Reprod Biol 2009;147:72-7. [Crossref] [PubMed]
- Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010;93:52-6. [Crossref] [PubMed]
- Alborzi S, Hamedi B, Omidvar A, et al. A comparison of the effect of short-term aromatase inhibitor (letrozole) and GnRH agonist (triptorelin) versus case control on pregnancy rate and symptom and sign recurrence after laparoscopic treatment of endometriosis. Arch Gynecol Obstet 2011;284:105-10. [Crossref] [PubMed]
- Chen YJ, Hsu TF, Huang BS, et al. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. Am J Obstet Gynecol 2017;216:582.e1-9. [Crossref] [PubMed]
- Jee BC, Lee JY, Suh CS, et al. Impact of GnRH agonist treatment on recurrence of ovarian endometriomas after conservative laparoscopic surgery. Fertil Steril 2009;91:40-5. [Crossref] [PubMed]
- Takamura M, Koga K, Osuga Y, et al. Post-operative oral contraceptive use reduces the risk of ovarian endometrioma recurrence after laparoscopic excision. Hum Reprod 2009;24:3042-8. [Crossref] [PubMed]
- Lee DY, Bae DS, Yoon BK, et al. Post-operative cyclic oral contraceptive use after gonadotrophin-releasing hormone agonist treatment effectively prevents endometrioma recurrence. Hum Reprod 2010;25:3050-4. [Crossref] [PubMed]
- Cucinella G, Granese R, Calagna G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Arch Gynecol Obstet 2013;288:821-7. [Crossref] [PubMed]
- Yamanaka A, Hada T, Matsumoto T, et al. Effect of dienogest on pain and ovarian endometrioma occurrence after laparoscopic resection of uterosacral ligaments with deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol 2017;216:51-5. [Crossref] [PubMed]
- Zhu S, Zhu Y, Liu Y, et al. Comparison of Outcomes of Different Postoperative Hormone Therapy in the Treatment of Ovarian Endometriosis: A Brief Report. Adv Ther 2018;35:857-63. [Crossref] [PubMed]
Cite this article as: D’Alessandro G, Barra F, Tantari M, Ferrero S. Hormonal treatments for preventing recurrence of endometriomas. Gynecol Pelvic Med 2020;3:20.