Minimally invasive surgery in rectopexy at the time of sacrocolpopexy
Introduction
Anterior, apical, and posterior vaginal wall defects are usually combined in pelvic organ prolapse (POP). In an epidemiologic cohort study in which 395 women identified, reported types and frequencies of defects were 40% for anterior compartment, 7% for posterior compartment only, 6% for apex only, 16% for anterior and posterior compartments, 9% for anterior compartment and apex, 5% for posterior compartment and apex, and 18% for all three compartments (1). Besides, to occur with other pelvic support defects, posterior vaginal defects may be associated with rectocele, sigmoidocele, and enterocele. POP often coexists with internal rectal prolapse or external rectal prolapse (ERP). In physical examination, distinguishing these entities are difficult. Besides, vaginal topography does not reliably predict the position of the associated viscera on pelvic floor fluoroscopy in POP patients (2). In addition to these diagnosis difficulties, Fenner found that the degree of anatomic distortion did not always correlate with functional impairment (3). To make things more complex, Altman et al. showed 15–60% of total vaginal prolapse cases also had some degree of rectal prolapse present (4).
Vaginal childbirth, advancing age, and increasing body mass index are the most common risk factors for any form of POP. Besides, chronically elevated intra-abdominal pressure and collagen vascular disease are other known risk factors (5,6). Hysterectomy may be involved in apical prolapse but it is not clear as a risk factor for posterior vaginal defects (5-7). Posterior POP may associate symptoms such as constipation, splinting, pelvic pressure, fecal incontinence, and sexual and/or defecatory dysfunction. Increased symptomatology does not necessarily with increased prolapse severity (8-11).
The combination of clinical findings of posterior wall defects and posterior vaginal bulge during straining and palpation of breaks in the rectovaginal fascia on rectovaginal examination make the diagnosis for posterior POP. In addition to the history and physical examination, imaging which is not routinely used in the evaluation of posterior wall defects might be a supportive diagnostic tool for patients whose symptoms are not consistent with examination findings or who have recurrent posterior vaginal wall defects. Therefore, there is no standardized method of establishing a radiological diagnosis of a rectocele; however, several imaging techniques such as defecography, contrast studies of the bowel, and magnetic resonance imaging (MRI) have been used. In addition to the imaging, there are some physiology ancillary tests such as colon transit study, endoanal ultrasound, anorectal manometry, and pudendal nerve latency.
Material and methods; treatment modalities for posterior POP
If the patient has symptomatic prolapse than treatment should be considered. There are 3 alternatives for treatment such as expectant management, nonsurgical options (pessary, physical therapy), and surgery. The patient should be counseled regarding the potential outcomes of these options. It is crucial to determine what the patient wants and expects from any intervention.
If the primary complaint is constipation, the patient’s dietary modifications should be considered as a first step. The patient should be encouraged to increase fluid intake, consume more fiber, and use laxatives. Rectocele repair might not be an option for women who describe life-long infrequent bowel movements (less than one per week) and the absence of a daily urge to defecate.
As aforementioned, the extent of the prolapse does not necessarily correlate with the extent of bowel symptoms. If the patient does not have bulge as a primary complaint, and defecatory dysfunction or fecal incontinence is her main complaint, ancillary testing should be pursued based on the woman’s complaints. In this scenario, surgical correction of a perineal body defect or rectocele may improve her symptoms, but it may not correct them (12-15). In a recent, nationwide longitudinal cohort study with 3,515 women who underwent POP surgery (16). In the same study, Karjalainen et al. concluded that obstructed defecation symptoms are dependent on the posterior wall anatomy. Besides, obstructed defecation symptoms improved more in women undergoing posterior compartment procedures compared with women undergoing repair of other compartments (16). Surgical repair tends to improve outlet dysfunction, where the stool gets trapped in the rectocele but does not address issues such as slow transit constipation, which can be associated with abdominal bloating.
Expectant management
In a longitudinal study of postmenopausal women who enrolled in the Women’s Health Initiative estrogen and progestin trial concluded that grade 1 (Baden Walker) posterior vaginal prolapse had a regression rate of 22 percent per 100 woman-years annually. However, grades 2–3 stages were unlikely to regress (17). Therefore, for mildly symptomatic women, observation with yearly examinations is appropriate. Surgical interventions for asymptomatic patients are not indicated but pelvic floor muscle exercises can be recommended. If the patient develops vaginal erosions which could not be treated with conservative methods, hydronephrosis due to urethral kinking, or obstructed urination or defecation, expectant management should be discontinued and replaced with appropriate treatment methods.
Non-surgical options
Pessary
Supportive and space-occupying are two main categories for pessaries. In a prospective, observational study, Clemons et al. concluded that seventy-three women (73%) with symptomatic POP had a successful pessary fitting trial. There were 2 risk factors for unsuccessful pessary fitting trial, a wide vaginal introitus, and a short vaginal length (18).
Pelvic floor muscle training
The purpose of pelvic floor muscle training is to increase the strength and endurance of the pelvic muscles. Therefore, it ameliorates support for pelvic organs. Its effectiveness has been shown in the treatment of urinary and fecal incontinence, but its role in the treatment of prolapse is unclear (19). Although there are minimal adverse effects, its cost can be seen as the main drawback. An experienced physical therapist or nurse practitioner may apply the most effective therapy with one to two visits per week for 8 to 12 weeks, with ongoing maintenance exercises in a supervised program. Advanced prolapse is unlikely to resolve with physical therapy alone.
Surgery
If surgical management is pursued, constipation should be managed in women with posterior wall prolapsed to avoid progression of the prolapse or recurrence.
The preoperative examination before the surgical admission should be done by the surgeon to find any evidence of an enterocele, sigmoidocele, or associated apical defects. If the pelvic examination does not support the patient’s symptoms or there is significant defecatory dysfunction, then pelvic floor MRI or defecation cystoproctography may reveal one or more of these other defects, or occult rectal prolapse or intussusception. If such defects are detected, the surgeon should counsel the patient about possible management options.
In some trials, bowel preparation before gynecologic surgery does not improve visualization, also in the transvaginal approach, a bowel preparation may contaminate the operative field (20-22).
The goal of rectocele repair is to relieve symptoms relevant to the anatomic support defect in the posterior vaginal wall. This repair can be performed by transvaginally with the patient in the dorsal lithotomy position, or endoanally with the patient in a prone or jack-knife position. In the transvaginal route, the traditional posterior colporrhaphy and the site-specific repairs are 2 main methods and their success rate is 76–96% and 82–100%, respectively (23,24). A perineorrhaphy, when indicated, completes the vaginal approach to a rectocele repair.
In addition to widely used surgical techniques for posterior compartment defects repair, graft augmentation and endorectal repair are other surgical options. The colorectal surgeons typically operate on the distal rectocele from the endoanal or endorectal approach. For women with an absent perineal body, anal sphincteroplasty is required and needs extensive dissection and repair.
Minimally invasive rectopexy during apical support treatment
Ventral rectal defect repair utilizing mesh
In most cases, posterior POP is secondary to loss of apical support or in some cases after correcting the apical support if the posterior wall is restored without a separate rectocele repair. The sacrocolpopexy is a procedure that attaches mesh to the anterior and posterior surfaces of the vagina to suspend the apex of the vagina to the sacral promontory. An open or minimally invasive approach may be performed when the rectocele is accompanied by apical prolapse. This article is mainly focused on the need for rectopexy during the apical vaginal repair.
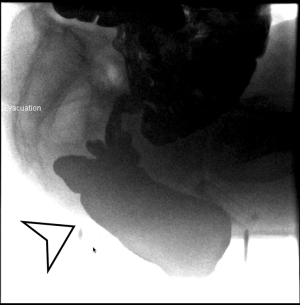
The pelvis is examined first, the end-to-end anastomosis (EEA) sizer vaginal manipulator is used as for vaginal manipulation (Figure 1). The sigmoid is reflected in the peritoneum and mesentery to expose rectovaginal space. The vagina is positioned anteriorly, and the posterior vaginal wall is dissected from the ventral rectum to the pelvic floor.
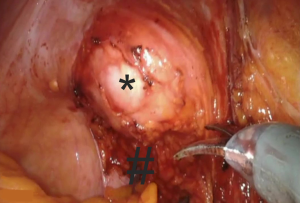
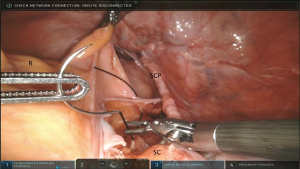
Digital palpation of the distal rectum is performed to determine the extent of the dissection. A mesh is secured to the ventral rectal serosa with 3 interrupted permanent (2-0 silk) sutures (Figure 2).
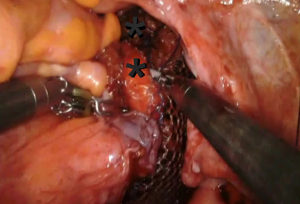
The anterior longitudinal ligament is exposed. Two 2-0 silk or Gortex sutures are placed into the anterior longitudinal ligament. The mesh is held at the desired location over the sacral promontory (Figure 3). The sutures are passed through both sheets of mesh in the access mesh is trimmed. The vaginal stand is used to minimize tension on the mesh as it is secured in the anterior longitudinal ligament. The vaginal stand is removed after securing the mesh to the anterior longitudinal ligament.
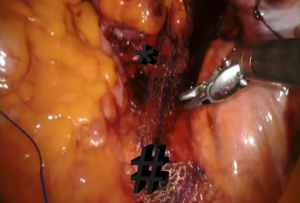
Posterior rectal defect repair with suturing technique
A peritoneal incision was made from just above the sacral promontory while preserving the right hypogastric nerve. This incision was extended along the right side of the rectum and over the bottom of the pouch of Douglas in an inverted J-shape. Denonvillier’s fascia was incised, and the rectovaginal septum was broadly opened. Its distal extent, usually 3–4 cm from the anal verge, was confirmed by digital rectal examination. It was sutured as distally as possible on the rectal muscular wall with six interrupted 2-0 non-absorbable sutures. The first suture will be placed in the pelvic floor to the rectal area; the second set of sutures approximates the rectal area to the S2–3 level of the longitudinal sacral ligament. Final sutures will be in the sacral promontorium level (Figure 4).
Discussion
In our clinic, clinical management and decision making follow the algorithm summarized below. If the patient has posterior defect identified based on POP quantification (POP-Q) exam, the next question will be to assess the correlation of gastrointestinal symptoms with the stage of prolapse. If there is a discordance between two, defecogram proceed it. Defecogram helps to identified good rectopexy candidates especially for high-grade internal rectocele and obstructive rectocele (Figure 5).
Considering high-grade internal rectocele present 60% of the patients have both obstructive recto-sigmoid and fecal incontinence, defecogram served a great volume of our pelvic floor disorder patients (25,26). During the manual exam, any abnormal anal sphincter tone findings will lead to anal ultrasound imaging to evaluate the level of sphincter deficiency.
Pre-operative surgical decision making summarized below in our clinic (Figure 6). Our perception to minimize to use of total mesh utilizing during combined surgeries. Based on pre-operative evaluation patients classified based on the predominant symptoms and stage of defects. If rectocele is more predominant compare to apical vaginal defect, then mesh preferred to use for ventral rectopexy. The high uterosacral suspension (HUSS) will be performed for apical vaginal repair in this patient group. However, in the cases of apical vaginal defect is more predominant to rectal prolapse; rectopexy will be proceeding it as a primary suturing technique so that mesh will be utilized for sacrocolpopexy.

Conclusions
Pelvic floor disorders are complex problems and all the anatomical structures are connected. It requires a very meticulous workup and strong collaboration between urogynecologists, gynecologic surgeon, colorectal surgeons, urologists, gastrointestinal specialists, physical therapists, radiologists, and dieticians. This combined effort projects a strong possibility to decrease the failure rate of our treatment modalities in prolapse patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-pfd-08/coif). The series “Minimally Invasive Treatment Modalities for Female Pelvic Floor Disorders” was commissioned by the editorial office without any funding or sponsorship. GSK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from Nov 2019 to Oct 2021.The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501-6. [Crossref] [PubMed]
- Kenton K, Shott S, Brubaker L. Vaginal topography does not correlate well with visceral position in women with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 1997;8:336-9. [Crossref] [PubMed]
- Fenner DE. Diagnosis and assessment of sigmoidoceles. Am J Obstet Gynecol 1996;175:1438-41. [Crossref] [PubMed]
- Altman D, Zetterstrom J, Schultz I, et al. Pelvic organ prolapse and urinary incontinence in women with surgically managed rectal prolapse: a population-based case-control study. Dis Colon Rectum. 2006;49:28-35. [Crossref] [PubMed]
- Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet 2007;369:1027-38. [Crossref] [PubMed]
- Vergeldt TF, Weemhoff M. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J 2015;26:1559-73. [Crossref] [PubMed]
- Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int Urogynecol J 2018;29:13-21. [Crossref] [PubMed]
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol 2001;185:1332-7. [Crossref] [PubMed]
- Weber AM, Walters MD, Ballard LA, et al. Posterior vaginal prolapse and bowel function. Am J Obstet Gynecol 1998;179:1446-9. [Crossref] [PubMed]
- Burrows LJ, Meyn LA, Walters MD, et al. Pelvic symptoms in women with pelvic organ prolapse. Obstet Gynecol 2004;104:982-8. [Crossref] [PubMed]
- Barber MD, Visco AG, Wyman JF, et al. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol 2002;99:281-9. [PubMed]
- Maher CF, Qatawneh AM, Baessler K, et al. Midline rectovaginal fascial plication for repair of rectocele and obstructed defecation. Obstet Gynecol 2004;104:685-9. [Crossref] [PubMed]
- Cundiff GW, Weidner AC, Visco AG, et al. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol 1998;179:1451-6. [Crossref] [PubMed]
- Singh K, Cortes E, Reid WM. Evaluation of the fascial technique for surgical repair of isolated posterior vaginal wall prolapse. Obstet Gynecol 2003;101:320-4. [PubMed]
- Porter WE, Steele A, Walsh P, et al. The anatomic and functional outcomes of defect-specific rectocele repairs. Am J Obstet Gynecol 1999;181:1353-8. [Crossref] [PubMed]
- Karjalainen PK, Mattsson NK, Nieminen K, et al. The relationship of defecation symptoms and posterior vaginal wall prolapse in women undergoing pelvic organ prolapse surgery. Am J Obstet Gynecol 2019;221:480.e1-e10. [Crossref] [PubMed]
- Handa VL, Garrett E, Hendrix S, et al. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol 2004;190:27-32. [Crossref] [PubMed]
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol 2004;190:345-50. [Crossref] [PubMed]
- Price N, Dawood R, Jackson SR. Pelvic floor exercise for urinary incontinence: a systematic literature review. Maturitas 2010;67:309-15. [Crossref] [PubMed]
- Siedhoff MT, Clark LH, Hobbs KA, et al. Mechanical bowel preparation before laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol 2014;123:562-7. [Crossref] [PubMed]
- Won H, Maley P, Salim S, et al. Surgical and patient outcomes using mechanical bowel preparation before laparoscopic gynecologic surgery: a randomized controlled trial. Obstet Gynecol 2013;121:538-46. [Crossref] [PubMed]
- Ballard AC, Parker-Autry CY, Markland AD, et al. Bowel preparation before vaginal prolapse surgery: a randomized controlled trial. Obstet Gynecol 2014;123:232-8. [Crossref] [PubMed]
- Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol 1993;36:976-83. [Crossref] [PubMed]
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 2013;4:CD004014 [Crossref] [PubMed]
- Kim AY. How to interpret a functional or motility test - defecography. J Neurogastroenterol Motil 2011;17:416-20. [Crossref] [PubMed]
- Alam NN, Narang SK, Köckerling F, et al. Rectopexy for Rectal Prolapse. Front Surg 2015;2:54. [Crossref] [PubMed]
Cite this article as: Unlu BS, Kilic GS. Minimally invasive surgery in rectopexy at the time of sacrocolpopexy. Gynecol Pelvic Med 2020;3:34.