
Narrative review of hysteroscopy and endometriosis treatment
Introduction
Endometriosis is defined as the presence of endometrial glands and stroma located outside the uterine cavity, occurring most commonly in the cul-de-sac, ovaries, and pelvic visceral and parietal peritoneum. It represents a chronic, inflammatory, eminently benign and estrogen dependent disease. This ectopic endometrial tissue can present glands and/or stroma, usually found in the pelvic region, but can be present in any location. It is called adenomyosis when tissue with endometrial characteristics is present in the myometrium.
The incidence of endometriosis is 10% to 15% of the female population at reproductive age, and may affect 25% of women with infertility. What worries most researchers is that it takes 7 to 10 years to be diagnosed (1).
The basis of the investigation of a patient with endometriosis and pain involves anatomical and functional knowledge of the pelvic and abdominal organs and characterization of the type of pain and its location. Colic-type pain may represent involvement of hollow muscle viscera. If it is central, retropubic, it may be related to uterine involvement, likely, adenomyosis, especially if associated with increased menstrual flow. Dysmenorrhea is now considered the greatest marker of endometriosis. If this colic pain, coinciding with menstruation, has associated diarrhea, dyschezia, and sometimes hematochezia, we may have bowel endometriosis, possibly in the rectosigmoid.
Adenomyosis is a benign uterine disease, characterized by the presence of glands and endometrial stroma in the uterine musculature. The presence of this tissue leads to hypertrophy and hyperplasia of the adjacent myometrium. Until very recently, the diagnosis was made only by anatomopathology, from the study of post-hysterectomy uterine specimens. Thus, the prevalence estimate of the disease has always been underestimated, since the diagnosis was only made in patients undergoing hysterectomy. In addition, the various studies that address this theme applied different histological criteria for diagnosis, making it difficult to carry out a more comprehensive assessment, leading to a huge variation in the prevalence of the disease in each study, which ranged from 5% to 70% (2).
Hysteroscopy is indicated in patients diagnosed with endometriosis when there is also infertility, in the investigation of intrauterine causes of dysmenorrhea and abnormal uterine bleeding. The investigation of the uterine cavity by hysteroscopy will only be indicated in cases where the treatment of choice leads to uterine preservation, if there is an indication for hysterectomy this investigation will not be necessary, except when there is suspicion of cervical or endometrial cancer.
In our evaluation, hysteroscopy is indicated in patients diagnosed with endometriosis when there are complaints of dysmenorrhea, abnormal uterine bleeding or findings of intrauterine changes in imaging tests or infertility.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-es-01/rc).
Methods
We performed a MEDLINE, Web of Sciences and Cochrane Library, from January to July 2020, using the term "Hysteroscopy " in combination with "endometriosis" OR "adenomyosis" OR "adenomyomectomy" OR "infertility”. We examined related references of selected papers as well as systematic reviews and meta-analyses. We considered articles published in English or Portuguese in peer-reviewed journals on medical conditions associated with endometriosis and hysteroscopic findings and treatment. Titles and abstracts of retrieved citations were screened, selected full-text articles were assessed for eligibility and data were extracted independently by two researchers (RL and BL); disagreement was resolved by discussion.
Discussion
Endometriosis is associated with a higher incidence of uterine diseases, with hysteroscopy being the main diagnostic and therapeutic tool in most cases. We can have a greater association between endometriosis and:
- Uterine polyps and fibroids;
- Uterine malformation;
- Endometritis;
- Uterine factor infertility (adenomyosis).
Like endometriosis, the pathophysiology of endometrial polyps is still not well understood, but both are associated with an estrogenic environment. The two diseases share genetic changes with greater expression of estrogen and aromatase receptors, in addition to dysregulation of apoptosis, with greater expression of bcl-2. Several studies have shown a higher incidence of endometrial polyps in patients with endometriosis, especially when infertility is associated (3). Kim et al. reported the prevalence of endometrial polyps in the endometriosis group and in the non-endometriosis group of 46.7% and 16.5%, respectively (4). Based on that information, it is important for endometriosis patients to undergo hysteroscopy to examine endometrial polyps, which can be resected together, especially for infertile patients. There are some data that suggest that there could be also links between endometriosis and endometrial cancer. Cancer-related genetic changes such as loss of heterozygosis, and altered methylation and expression patterns have been reported for endometriosis (5). Numerous endometrial cancer-associated genes, including PTEN and other genes in the Ingenuity “endometrial cancer pathway,” have been shown to be dysregulated in endometriosis (6-8). This is still under debate in the scientific community, due to the heterogeneity of the research and low quality of evidence.
In addition to endometrial polyps, patients with endometriosis appear to have a higher incidence of uterine fibroids. Once again, the estrogen route appears to be the link between the two diseases. Nezhat et al., from the surgical evaluation of patients who underwent myomectomy or hysterectomy for symptomatic myoma, found the presence of endometriosis, confirmed with biopsy, in 87.1% of the patients (9). Nicolaus et al. found 57 patients (25.6%) with endometriosis among 223 who underwent laparoscopic myomectomy for symptomatic fibroids. They identified that endometriosis was associated with infertility, nulliparity and smaller fibroid size (10). Because of the significant overlap of symptoms, it is often difficult to discern which pathology is responsible for the patient’s complaints. Thus, it is essential to properly investigate the patient with endometriosis, since the failure to identify a fibroid can keep the patient symptomatic even after surgical resection of the endometriosis. It is known that the submucosal myoma is the main one associated with symptoms, AUB, and hysteroscopy is the method of choice for the diagnosis and treatment of these lesions.
Hysteroscopy is an important tool in the evaluation of uterine morphology. Endometriosis is associated with obstructive anomalies and nonobstructive malformations, especially those concerning the septated uterus. According to Sampson’s theory, a change in uterine morphology with obstruction of the outflow would lead to a higher incidence of endometriosis due to a significant tubal reflux. Song et al. demonstrated that women with cervical atresia had an increased frequency of endometriosis (11). LaMonica et al. reported that the incidence of septated uterus in women with infertility and/or pelvic pain ranges from 27% to 37%, being significantly higher in women with endometriosis and mores so with stage IV disease (12). In an observational study of 52 patients with uterine malformation, Boujenah et al. identified a higher incidence of moderate and severe endometriosis (endometrioma and DIE) in infertile women with uterine malformations (13). In this study, there was no difference in relation to peritoneal endometriosis or the type of associated uterine malformation.
It cannot be forgotten that the internal orifice stenosis, diagnosed and treated in hysteroscopy, causes dysmenorrhea.
Thus, in patients with endometriosis and associated infertility, hysteroscopy allows the evaluation of the uterine cavity and the identification or exclusion of a morphological alteration, obstructive or not, concomitantly.
Chronic endometritis (CE) can be asymptomatic or have nonspecific symptoms such as intermenstrual bleeding, pelvic pain, dyspareunia, leukorrhea or intermittent cystitis. It is characterized by infiltration of plasma cells in the endometrial stroma, where they are not normally present, except in the menstrual period, in addition to dissociation of cell maturation, increased stromal cellularity and edema. It is a persistent inflammatory process, with a direct impact on endometrial quality, and important repercussions on the patient’s fertility. It has been identified in 12–46% of infertile patients, 30% of repeated implantation failures after in vitro fertilization-embryo transfer, 28% of unexplained infertility, and 12% of unexplained recurrent miscarriages (14-16). Cicinelli et al., based on post hysterectomy endometrial analysis of patients with stage IV endometriosis and without endometriosis, found a 2.7 greater risk of endometritis in patients with endometriosis. According to the authors, an etiopathogenetic link between CE and endometriosis may rely on altered uterine contractility. In women with CE, there is a decrease in the anterograde subendometrial contractions present during menses, which contribute to the forward emptying of menstrual blood, which thus facilitates retrograde reflux of menstrual bleeding through the fallopian tubes. This leads us to speculate that, according to Sampson’s theory, CE may represent a facilitating factor for the development of endometriosis (17).
Takebayashi et al., using endometrial immunohistochemical study (CD138) in patients with and without endometriosis undergoing hysterectomy, found chronic endometritis in 52.94% of the endometriosis group and 27.02% of the non-endometriosis group. The rate of chronic endometritis was analyzed at each stage of endometriosis. Chronic endometritis was found in 40.0% of stage I endometriosis, 50.0% of stage II, 70.0% of stage III, and 46.7% of stage IV. The presence of ovarian endometrioma showed no difference in the prevalence of CE in this study (18).
Higher bacterial contamination of menstrual blood and an increased endotoxin level in menstrual and peritoneal fluids have been found in women with endometriosis as compared with control women (19). The number of colony-forming units (CFU/mL) of Gardnerella, a-Streptococcus, Enterococci, and E. coli was statistically significantly higher in endometrial samples obtained from women with endometriosis than in controls.
CE can be focal or diffuse and the definitive diagnosis is made by anatomopathological study of the endometrial material, with or without immunohistochemical study. This biopsy can be done in several ways, being hysteroscopy the main method of choice, as it allows a direct view of the endometrial cavity, allowing the diagnosis of endometritis even if focal. The directed biopsy should be done, being the biopsy of the region with the greatest visual alteration.
The association of adenomyosis and endometriosis is very frequent. There is a constant debate in the literature as to whether they are different entities or just different sites of the same disease. Like endometriosis, the etiology of adenomyosis is not known, and shares some of the theories of the former (20-22).
The main theory is based in the rupture of the endometrial-myometrial layer, allowing the invasion of glands and endometrial stroma in the myometrium. This region is devoid of a submucosal layer, probably to facilitate trophoblastic invasion in the gestational period and, therefore, pro-inflammatory states or local trauma can weaken this site, leading to a rupture. There is no transition tissue between the endometrium and the myometrium, therefore, uterine curettages, cesarean sections and multiple pregnancies can lead to a rupture of the endometrial-myometrial interface, generating hyperplasia of the basal layer and infiltration in the injured myometrium (21-23). Likewise, uterine hyperperistalsis or dysperistalsis can lead to local fissures, favoring the development of the disease.
The immune system has also been described as causing adenomyosis, due to the action of activated T and B macrophages, which would produce antibodies and cytokines capable of weakening the endomyometrial interface and allowing the invagination of the basal layer (22).
There is evidence of a higher local estrogen concentration in patients with adenomyosis. The eutopic endometrium of patients with adenomyosis and endometriosis are sources of estrogen. Kitawaki and collaborators (24) analyzed the concentrations of estradiol in the topical endometrium (menstrual blood) and in the peripheral circulation of patients with adenomyosis, endometriosis and in those with normal menstrual cycle. The result found was a high concentration of estradiol in the menstrual blood of patients with adenomyosis, followed by those with endometriosis, and a low concentration in patients without disease. Blood levels showed no significant difference. These results, according to the authors, prove the local production of estrogens, which is corroborated by the expression of aromatase P450 in high concentrations in the endometrium of patients with adenomyosis, and its absence in the endometrium of disease-free patients (1,22).
Other hormonal changes have been described as inducing adenomyosis. In animal models, hyperprolactinemia led to the development of adenomyosis in rats. High rates of prolactin, together with the presence of steroid hormones, would be associated with degeneration of myometrial cells and, therefore, would favor the invasion of endometrial tissue in the adjacent myometrium (22,25-28). Some classes of antidepressant drugs are related to the onset of hyperprolactinemia, with the use of these substances being frequent in patients with adenomyosis, due to depression associated with chronic pelvic pain.
Adenomyosis shares with endometriosis the theory of origin from embryonic remains (1,29). Mullerian tissue present in the myometrium would differ from endometrial tissue. This theory corroborates the presence of adenomyotic tissue without continuity or proximity to the uterine cavity, close to serosa, for example. In addition, comparative analyzes between these foci of adenomyosis and the topical endometrium showed different compositions, suggesting different origins.
Other theories, similar to that of endometriosis, associate the development of the disease to the intramiometrial lymphatic spread of endometrial tissue, or through the transformation of stem cells into endometrial tissue. Endometrial regeneration in the menstrual cycle is associated with the presence of stem cells, which could lead to the development of endometrial tissue within the myometrium (30,31).
Junction zone (ZJ)
The transition layer from the endometrium to the myometrium is called the subendometrial myometrium or ZJ. This region shares greater similarities with the endometrium than with the myometrium. Its origin, like that of the endometrium, is Mullerian, and changes in its constitution and thickness during the phases of the menstrual cycle (1,21). Like the endometrium, its thickness is maximum around the 8th to the 16th day of the menstrual cycle. The use of hormonal contraceptives, GnRH analogues and menopause reduce the thickness of this region. ZJ can be absent in up to 20% of women.
The normal thickness of the ZJ is up to 6–8 mm, being well defined on magnetic resonance or transvaginal ultrasonography. In the presence of adenomyosis, this region is thickened >12 mm, corresponding to myometrial hyperplasia, which may contain endometrial cysts (1).
Analyzes of uterine contractility, outside the pregnancy period, showed that these contractions originate exclusively in the ZJ. The amplitude and frequency of this peristalsis is related to the phase of the menstrual cycle, but the control is still not well understood (32). Another function of ZJ is to allow trophoblastic invasion to occur properly, which may be related to abortion, preeclampsia, restricted intrauterine growth and premature birth, when there are changes in this region.
Classification (Table 1)

Full table
Adenomyosis can be divided into focal—adenomyoma—and diffuse. Adenomyoma is characterized by a myometrial mass, with poorly defined contours, which differs from leiomyoma because it does not present a pseudocapsule or a cleavage plane with the myometrium. There is also the adenomyomatous polyp and cystic adenomyosis (32). The first corresponds to an intracavitary polypoid formation with muscle cell composition and endometrial foci. Cystic adenomyosis consists of cystic formations with hematic content surrounded by myometrium, being more frequent in adolescents or young adults. Cysts change in size throughout the menstrual cycle, and there may be clinical resistance to the use of hormonal treatment.
There are several proposals to classify the disease. The most widely used is the one that uses as a parameter the degree of invasion of ectopic endometrial tissue in the myometrium. It is classified as superficial when the invasion is less than 40%, intermediate when it reaches 40 to 80% and deep when it exceeds 80% penetration.
Adenomyosis is associated with infertility for a few main reasons (33-37):
- Uterine peristalsis disorder and sperm transport.
- Abnormalities in the uterine cavity.
- Change in endometrial function and receptivity.
The direction of uterine contractions has a fundamental factor in the reproductive process, as it allows the proper transport of sperm towards the egg. Two main moments in the menstrual cycle mark uterine peristalsis: Menstrual period, where the sense of contractions is fundus-cervix, to facilitate the flow of endometrial desquamation; Periovulatory period, with cervical-fundus contractions, to allow adequate transport of sperm, directed to the tubal ostium ipsilateral to the dominant follicle. Uterine peristalsis originates in the junctional zone, a region affected by adenomyosis, generating hyperperistalsis and uterine dysperistalsis, in addition to increased intrauterine pressure. In this way, sperm transport is compromised, leading to infertility (25).
The presence of adenomyoma (focal adenomyosis) in the uterine cavity, as with submucosal fibroids, leads to distortion in the architecture of this region, which can lead to infertility by obstructing the internal orifice and/or tubal ostia, making it difficult or impossible for sperm to pass (37).
The endometrial response seems to be altered in patients with adenomyosis. The high concentration of P450 aromatase in the endometrium of these patients, as previously discussed, is related to the worse response in assisted reproduction methods. In addition, inflammatory markers, such as interleukin 6 and 10 (IL-6, IL-10), and free radicals are in high concentrations in this region, promoting a toxic effect on the embryo. Some adhesion molecules (integrins, selectins and cadherins), which are essential for embryo implantation, are at lower concentrations in patients with adenomyosis when compared to patients free of the disease. The HOXA 10 gene expression, associated with endometrial receptivity and embryonic development, also seems to be altered in these patients in relation to fertile patients (37). The unfavorable intrauterine environment is associated with a higher risk of miscarriage in patients with adenomyosis.
The identification of uterine adenomyosis in hysteroscopy depends on the phase of the cycle in which the exam is performed, should be performed in the recent post-menstrual period and the lesion must be affecting the uterine cavity. As adenomyosis is a myometrial disease, diagnosis by hysteroscopy will only be possible when it is superficial or, when myometrial, it has a portion in the uterine cavity. For this reason, the diagnosis of adenomyosis in hysteroscopy makes the surgeon require imaging exams for myometrial evaluation seeking to assess the extent of adenomyosis, which may be magnetic resonance or 3D ultrasound. In this way, it will be possible to decide the access route for treatment, hysteroscopic or abdominal, knowing that the hysteroscopic is indicated only in superficial adenomyosis and the abdominal in others.
Hysteroscopy to adenomyosis can present itself as a bluish-colored cystic formation, which, when sectioned, has a chocolate content inside.
Hysteroscopic treatment
The presence of polyps, fibroids and uterine malformations, as previously mentioned, is often associated with endometriosis. Uterine polyps should be removed by hysteroscopy, as well as fibroids with a submucosal component, usually those most associated with AUB (38). Some uterine malformations can also be treated hysteroscopically, such as uterine septa for example. Hysteroscopic myomectomy should be indicated according to some hysteroscopic criteria. The Lasmar or STEP-W classification (39,40) is able to predict the complexity and viability of the procedure (Tables 2,3).

Full table

Full table
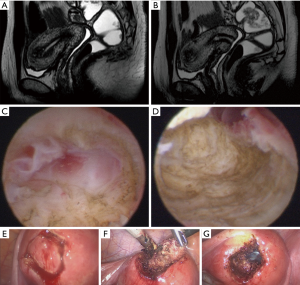
Hysteroscopic treatment of adenomyosis is possible when the disease is located close to the uterine cavity. Sometimes in outpatient hysteroscopy, drainage of the adenomyotic cyst and partial resection of its capsule is possible, using biopsy-punch, with improvement of dysmenorrhea. Always depending on the patient’s pain tolerance (Figure 1).
When adenomyosis is superficial and focal and the patient wants pregnancy, resection of the lesion with mono or bipolar resectoscope is possible. The procedure may or may not be preceded by the GnRH analog, or it may be performed at the beginning of the first phase of the cycle, with a newly peeled endometrium. With the semi-circle handle, the most nodular area is “palpated”. With the movement of the energized loop towards the cervix of the uterus, the lesion is removed, with all its depth and extension (Figure 2). In those with no pregnancy desires, endometrial ablation may be an option as well as the association of ablation with the placement of levonorgestrel IUDs.
Laparoscopic, robotic or vaginal hysterectomy assisted by laparoscopy has become an excellent alternative to conventional laparotomy treatment, bringing with it all the benefits of endoscopic surgery in terms of length of stay, morbidity and recovery of patients. Hysterectomy is an option for patients with constituted offspring. In specific situations where it is necessary to preserve fertility, laparoscopy for resection of adenomyosis can be performed, being a great challenge in cases of diffuse and extensive extension. In these cases, an attempt is made to resect as much tissue as possible, always keeping in mind the need to preserve uterine function, and it is often impossible to resect the entire disease (Figure 3).
For adenomyoma the resection of the nodules is performed as in the case of myomectomy, however, in these cases, the cleavage plan is less defined, requiring more experience from the surgeon and an adequate analysis of the path chosen for the approach. Sometimes it is necessary to monitor hysteroscopic resection of adenomyosis by laparoscopy at the same time. We usually choose this complementation when there is infiltration up to the proximity of the uterine serosa. Another option is to start the procedure by performing a hysteroscopic resection to a certain myometrial depth and, in the same surgical procedure, to perform a laparoscopic resection of the rest of the infiltrated area (Figure 3).
Pregnancies that occur after a deep surgical approach to adenomyosis have a higher incidence of miscarriage, uterine ruptures, which can be silent, and a higher incidence of anomalous placentation. The latter are comparable to post-cesarean section findings and myomectomy.
Final considerations
Hysteroscopy brings important collaboration in the investigation and treatment of intrauterine diseases in patients with endometriosis who have no indication for hysterectomy.
The investigation of causes for infertility, dysmenorrhea and abnormal uterine bleeding may originate in the uterine cavity, as well as the identification of uterine malformations.
Our guidance is to have hysteroscopy performed before laparoscopic or robotic surgery in patients with endometriosis. If office hysteroscopy is not possible, it should be performed at the beginning of the same surgery to treat endometriosis. Further studies must be performed to evaluate the origin and better treatment of the disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Andrea Tinelli) for the series “Endometriosis Surgery” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-es-01/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-2020-es-01/coif). The series “Endometriosis Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abbott JA. Adenomyosis and Abnormal Uterine Bleeding (AUB-A)-Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol 2017;40:68-81. [Crossref] [PubMed]
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus—revisited. Am J Obstet Gynecol 1972;112:583-93. [Crossref] [PubMed]
- Zhang YN, Zhang YS, Yu Q. Higher Prevalence of Endometrial Polyps in Infertile Patients with Endometriosis. Gynecol Obstet Invest 2018;83:558-63. [Crossref] [PubMed]
- Kim MR, Kim YA, Jo MY. High frequency of endometrial polyps in endometriosis. J Am Assoc Gynecol Laparosc 2003;10:46-8. [Crossref] [PubMed]
- Kokcu A. Relationship between endometriosis and cancer from current perspective. Arch. Gynecol. Obstet 2011;284:1473-9. [Crossref] [PubMed]
- Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci 2011;18:229-51. [Crossref] [PubMed]
- Dentillo DB, Meola J, Ferriani RA. Common dysregulated genes in endometriosis and malignancies. Rev Bras Ginecol Obstet 2016;38:253-62. [Crossref] [PubMed]
- Painter JN, O’Mara TA, Morris AP, et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med 2018;7:1978-87. [Crossref] [PubMed]
- Nezhat C, Li A, Abed S, et al. Strong Association Between Endometriosis and Symptomatic Leiomyomas. JSLS 2016;20:e2016.00053.
- Nicolaus K, Bräuer D, Sczesny R. Unexpected coexistent endometriosis in women with symptomatic uterine leiomyomas is independently associated with infertility, nulliparity and minor myoma size. Arch Gynecol Obstet 2019;300:103-8. [Crossref] [PubMed]
- Song X, Zhu L, Ding J. Clinical characteristics of congenital cervical atresia and associated endometriosis among 96 patients. Int J Gynaecol Obstet 2016;134:252-5. [Crossref] [PubMed]
- LaMonica R, Pinto J, Luciano D. Incidence of Septate Uterus in Reproductive-Aged Women With and Without Endometriosis. J Minim Invasive Gynecol 2016;23:610-3. [Crossref] [PubMed]
- Boujenah J, Salakos E, Pinto M, et al. Endometriosis and uterine malformations: infertility may increase severity of endometriosis. Acta Obstet Gynecol Scand 2017;96:702-6. [Crossref] [PubMed]
- Féghali J, Baker J, Mayenga JM, et al. Systematic hysteroscopy prior to in vitro fertilization. Gynecol Obstet Fertil 2003;31:127-31. [PubMed]
- Polisseni F, Bambirra EA, Camargos AF. Detection of chronic endometritis by diagnostic hysteroscopy in asymptomatic infertile patients. Gynecol Obstet Invest 2003;55:205-10. [Crossref] [PubMed]
- Cicinelli E, Resta L, Nicoletti R, et al. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invasive Gynecol 2005;12:514-8. [Crossref] [PubMed]
- Cicinelli E, Trojano G, Mastromauro M, et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil Steril 2017;108:289-295.e1. [Crossref] [PubMed]
- Takebayashi A, Kimura F, Kishi Y, et al. The association between endometriosis and chronic endometritis. PLoS One 2014;9:e88354. [Crossref] [PubMed]
- Khan KN, Kitajima M, Hiraki K, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril 2010;94:2860-3.e1. [Crossref] [PubMed]
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A Clinical Review of a Challenging Gynecologic Condition. J Minim Invasive Gynecol 2016;23:164-85. [Crossref] [PubMed]
- Benagiano G, Brosens I, Habiba M. Adenomyosis: a life-cycle approach. Reprod Biomed Online 2015;30:220-32. [Crossref] [PubMed]
- Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril 2012;98:572-9. [Crossref] [PubMed]
- Taylor AH, Kalathy V, Habiba M. Estradiol and tamoxifen enhance invasion of endometrial stromal cells in a three-dimensional coculture model of adenomyosis. Fertil Steril 2014;101:288-93. [Crossref] [PubMed]
- Kitawaki J, Noguchi T, Amatsu T, Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 1997;57:514e519.
- Kissler S, Zangos S, Wiegratz I. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann N Y Acad Sci 2007;1101:38-48. [Crossref] [PubMed]
- Yamashita M, Matsuda M, Mori T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci 1997;60:1437-46. [Crossref] [PubMed]
- Fiçicioğlu C, Tekin HI, Arioğlu PF. A murine model of adenomyosis: the effects of hyperprolactinemia induced by fluoxetine hydrochloride, a selective serotonin reuptake inhibitor, on adenomyosis induction in Wistar albino rats. Acta Eur Fertil 1995;26:75-9. [PubMed]
- Bedaiwy MA, Dahoud W, Skomorovska-Prokvolit Y. Expression of progesterone receptor isoforms-A and-B in adenomyosis. Fertil Steril 2013;100:S387. [Crossref]
- Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update 2014;20:386-402. [Crossref] [PubMed]
- Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:511-21. [Crossref] [PubMed]
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008;1127:106-15. [Crossref] [PubMed]
- Tocci A, Greco E, Ubaldi FM. Adenomyosis and ‘endometrial-subendometrial myometrium unit disruption disease’ are two different entities. Reprod Biomed Online 2008;17:281-91. [Crossref] [PubMed]
- Koike H, Egawa H, Ohtsuka T. Correlation between dysmenorrheic severity and prostaglandin production in women with endometriosis. Prostaglandins Leukot Essent Fatty Acids 1992;46:133-7. [Crossref] [PubMed]
- Bazot M, Fiori O, Darai E. Adenomyosis in endometriosis—prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 2006;21:1101-2, author reply 1102-3. [Crossref] [PubMed]
- Kunz G, Beil D, Huppert P. Adenomyosis in endometriosis—prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 2005;20:2309-16. [Crossref] [PubMed]
- Kissler S, Hamscho N, Zangos S. Uterotubal transport disorder in adenomyosis and endometriosis - a cause for infertility. BJOG 2006;113:902-8. [Crossref] [PubMed]
- Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod Biomed Online 2012;24:35-46. [Crossref] [PubMed]
- Lasmar RB, Lasmar BP. The role of leiomyomas in the genesis of abnormal uterine bleeding (AUB). Best Pract Res Clin Obstet Gynaecol 2017;40:82-8. [Crossref] [PubMed]
- Lasmar RB, Lasmar BP, Celeste RK. A new system to classify submucous myomas: a Brazilian multicenter study. J Minim Invasive Gynecol 2012;19:575-80. [Crossref] [PubMed]
- Lasmar RB, Barrozo PR, Dias R. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment--preliminary report. J Minim Invasive Gynecol 2005;12:308-11. [Crossref] [PubMed]
Cite this article as: Lasmar RB, Lasmar BP. Narrative review of hysteroscopy and endometriosis treatment. Gynecol Pelvic Med 2021;4:7.




