
Expectant management of a viable Cesarean scar pregnancy complicated by uterine dehiscence and massive hemorrhage: a case report and literature review
Introduction
Cesarean scar pregnancy (CSP) is defined as pregnancy that implants within the fibrous tissue of an existing Cesarean section scar (1-4). It is a rare complication of a previous Cesarean section, with the incidence of 1/1,800–1/2,216 of all Cesarean deliveries (1). Up to 72% of CSP occur in patients who have had two or more previous Cesarean sections (5,6). First reported by Larsen and Solomon in 1978 (7), the incidence of CSP has been rising due to the increasing rate of Cesarean sections worldwide (1), and with increased early diagnosis by first trimester ultrasound (8).
The current report presents a unique case of expectant management of CSP complicated by uterine dehiscence that resulted in a successful live birth at 27 weeks 6 days gestation, with subsequent post-partum hemorrhage managed without hysterectomy. We present the following case in accordance with the CARE Reporting Checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-19-39/rc) (9).
The case
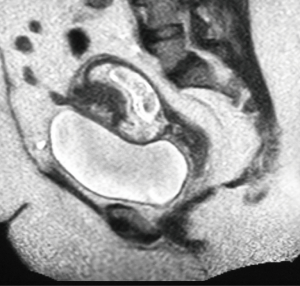
The timeline of events is depicted in Figure 1. The patient is a 31-year-old G6P2A3L2 female with a planned and wanted pregnancy who was diagnosed with CSP by ultrasound performed at 10 weeks and 5 days gestation (Figure 2). The ultrasound revealed the gestational sac to be in the anterior lower uterine segment with possible placental invasion through the myometrium towards the bladder. An MRI of the pelvis was performed at 11 weeks gestation to assess for placental invasion, and revealed CSP with partial dehiscence of the previous Cesarean section scar, and suspicion of focal placenta accreta (Figure 3).
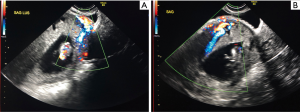
The patient’s obstetrical history included 2 previous Cesarean sections in 2011 and 2013 with live births, followed by three spontaneous abortions in the first trimester that were managed expectantly. She had a previous history of postpartum hemorrhage requiring 2 units of pRBC. The first Cesarean section was performed due to second stage labour dystocia secondary to cephalopelvic disproportion. The second Cesarean section was an elective repeat procedure.
Following extensive counselling throughout pregnancy by multiple obstetricians regarding treatment options and the risk of catastrophic events, she declined to terminate the pregnancy and chose to proceed with expectant management to reach viability. She experienced three episodes of hemorrhage at 4, 7, and 12 weeks gestation. At that time, the pregnancy was followed closely in a tertiary care centre by Maternal Fetal Medicine specialists with bimonthly ultrasounds. The goal was to deliver by elective Cesarean section at 35 weeks with the possibility of Cesarean hysterectomy.
She then presented to Labour and Birth at 9:54 with confirmed preterm prelabour rupture of membranes (PPROM) at 27 weeks and 6 days gestation that occurred at 8:00. The patient did not report any bleeding or abdominal pain. She was found to have pre-eclampsia with BP 145/71 and her pregnancy was further complicated by placenta previa, morbid obesity with BMI of 56.8, gestational diabetes, and Candidiasis at the previous skin incision site. She was admitted to hospital, placed on Mercer protocol (10), given 1 dose of antenatal corticosteroids on the day of admission, and NICU was consulted.
Hours after admission, she experienced uterine scar dehiscence that presented as an acute isolated episode of orthostatic hypotension with BP 92/57, pulse of 118 and minimal vaginal bleeding with the absence of abdominal pain at 16:35. The blood pressure normalized and the patient remained hemodynamically stable. An urgent ultrasound revealed a large collection of fluid suspicious for placental abruption with the fetus in frank breech presentation within the uterus. The umbilical cord was presenting in the lower uterine segment. The fetal heart rate remained normal. Magnesium sulfate for neuroprotection was promptly administered, and the patient gave consent for Cesarean section and a possible hysterectomy. After a thorough discussion about the risks of possible future pregnancy, the patient continued to have a strong desire to preserve fertility.
An emergent Cesarean section was performed under general anesthesia at 27 weeks 6 days gestation at 21:52 in the main operating room. During the procedure, uterine dehiscence of the previous uterine scar was identified. Serosa and uterovesical peritoneal folds contained 200 mL of blood. The Foley catheter was draining clear urine indicating no evidence of bladder involvement. Anterior placenta previa was also present. A transverse uterine incision was made cephalad to the previous scar and a live male infant was delivered using breech extraction at 22:02. The NICU team was present at the delivery.
The infant initially cried at delivery and delayed cord clamping was performed. The infant then experienced cardiopulmonary depression and required CPR, intubation, and ventilation. Birth weight was 1.200 kg and the APGAR scores were 1, 3, and 4 at 1, 5, and 10 minutes respectively. Cord arterial pH was 7.32. The infant was admitted to NICU.
The placenta separated easily during manual removal. There was excessive bleeding from the lower uterine segment and a series of figure-of-eight sutures were placed. Estimated blood loss was 1,200 mL and the patient had excellent abdominal and vaginal hemostasis. She received intraoperative cell salvage and 1 unit of pRBC and was then transferred to the recovery room in a stable condition.
At 2:10 on post-operative day 1, the patient became hemodynamically unstable with severe pallor, altered level of consciousness, tachycardia, hypotension, poor uterine tone, and an additional blood loss of 500 mL. The patient was brought back to the operating room at 2:27 and gave consent for the placement of a Bakri balloon and a possible hysterectomy. She had a massive postpartum hemorrhage in the operating room that was complicated by hemorrhagic shock and disseminated intravascular coagulopathy (DIC) with a fibrinogen level of 0.8 g/L and hemoglobin of 62 g/L. This was managed with uterotonics, placement of a Bakri balloon, vaginal packing, IV fluids, tranexamic acid, massive transfusion protocol, and embolization of bilateral internal iliac and uterine arteries with gelfoam slurry at 5:40. Vaginal bleeding subsided and the patient was then transferred to the intensive care unit (ICU).
The total estimated blood loss was 7,000 mL. She required a total transfusion volume of 13 units of pRBC, 4 units of FFP, 6 units of platelets, and 2 units of cryoprecipitate. Her postpartum course was complicated by small bilateral pulmonary embolisms (PE), acute kidney injury secondary to hypovolemic shock, thrombocytopenia with platelets of 17×103/mm3, pulmonary edema, and postpartum depression. She was discharged in stable condition on post-op day 7. The patient was counselled to avoid any future pregnancies.
The infant’s course in NICU was complicated by metabolic acidosis, pulmonary insufficiency, 2 episodes pneumothorax, respiratory distress syndrome (RDS), apnea of prematurity, pneumonia, evolving chronic lung disease, patent ductus arteriosus (PDA), intraventricular hemorrhage (IVH), possible sepsis, anemia of prematurity, jaundice, and hyponatremia. The length of stay in NICU was 74 days and the infant is currently doing well.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
Cesarean section is the most common inpatient surgical procedure in Canada with rates ranging from 18.5% to 35.3% of all pregnancies in 2016–2017 (11). CSP, also known as Cesarean ectopic pregnancy, occurs in 0.15% of patients who had a previous Cesarean section (5). The reported interval between a Cesarean section and subsequent CSP varies from 6 months to 12 years (12). The number of CSP case reports has increased from 19 cases reported between 1978 and 2002 (13) to 751 cases reported by 2012 (14).
Types of CSP
Two types of CSP have been proposed by Vial et al. (15). The patient presented in this case had Type 1 CSP. Type 1 CSP pregnancy involves implantation of the amniotic sac on the Cesarean section scar (2). It may progress in two ways: towards the uterine cavity or towards the cervico-isthmic space (16). This type of CSP is more likely to continue to viability and subsequently result in live birth (2). Type 2 CSP pregnancy involves deep implantation into the Cesarean scar defect and progression towards the myometrium and serosal layers of the uterus (4). Patients with Type 2 CSP require immediate management due to high risk of life-threatening uterine rupture and intraperitoneal hemorrhage in the first trimester (1).
Risk factors
Risk factors for CSP include two or more previous Cesarean deliveries, in vitro fertilization (IVF), previous manual removal of placenta, and trauma from previous other uterine surgeries such as dilatation and curettage (D&C), myomectomy (1), hysteroscopy, and metroplasty (2).
Pathogenesis
The blastocyst implants into a fully healed hysterotomy scar or a uterine niche. Multiple Cesarean sections increase the surface area of a uterine scar, which increases the risk of implantation into the scar. Furthermore, improper healing of the Cesarean incision due to fibrosis or poor vascularization can lead to thinning of the anterior uterine wall and create a defect in the scar called a uterine niche. A uterine niche is found in 24–70% of women with at least one previous Cesarean delivery (17) and can be diagnosed prior to pregnancy by ultrasound (18). Once implanted, the gestational sac invades through the niche into the uterine scar and has worse outcomes than a sac that implants into a fully healed scar (3).
Diagnosis
Early diagnosis of CSP is important for directing treatment, decreasing the risk of morbidity and mortality, and preserving fertility (1). CSP has a variable presentation and typically presents at 7.5±2.5 weeks of pregnancy (18). In the Rotas et al. review of 57 patients with CSP, 36.8% of patients were asymptomatic, 38.6% presented with painless vaginal bleeding, 15.8% presented with abdominal pain and vaginal bleeding, and 8.8% only had abdominal pain (18). In asymptomatic patients, diagnosis of CSP is usually made incidentally at the time of ultrasound examination (3). The review states that 13.6% of CSP were misdiagnosed as spontaneous abortion, cervical pregnancy, or low intrauterine pregnancy (18). A CSP may also present with a severe hemorrhage during D&C (12).
Transvaginal ultrasound with Doppler flow studies is the first-line imaging modality for diagnosis of CSP with a sensitivity of 84.6% (95% CI, 0.763–0.905) (18). Doppler flow studies can be used to differentiate between a viable and non-viable CSP (16). The sonographic criteria for the diagnosis of CSP are described by Jurkovic (6) as follows:
- Empty uterus and empty cervical canal (6);
- Development of gestational sac in the anterior wall of the isthmic portion of the uterus (6);
- Discontinuity on the anterior wall of the uterus running through the amniotic sac on sagittal plane (16);
- Diminished or absent healthy myometrium between bladder and gestational sac (6);
- Doppler examination demonstrating high velocity with low impendence peri-trophoblastic vascular flow surrounding the gestational sac (6).
Since transvaginal ultrasonography cannot provide a definitive diagnosis in all cases, contrast-enhanced MRI may be used as an adjunct for diagnosis of CSP in select patients (19). MRI has superior soft-tissue characterization and may be used to determine the exact location of the gestational sac. If the possibility of a morbidly adherent placenta (MAP) cannot be excluded with transvaginal ultrasounds, then MRI may be used to assess for placental invasion (20) and to assist with pre-operative planning (3). Cystoscopy may also be used to assess for bladder invasion (3).
Complications
Extensive counselling early in pregnancy is essential. The complication rate in CSP can be as high as 44.1% (21), which includes uterine dehiscence, uterine rupture, life-threatening hemorrhage from the implantation site (7), hemorrhagic shock (22), need for blood transfusion, coagulation disorders (13), need for uterine or iliac artery embolization (18), preterm labour (3), arteriovenous malformation (22), hysterectomy with subsequent loss of future fertility (23), and maternal and fetal death (1). It may be reasonable to wait until the CSP can be definitively characterized as Type 1 or Type 2, since that substantially affects the prognosis.
Furthermore, CSP may be a precursor to MAP. Timor-Tritsch et al. present evidence that CSP and MAP may be on a continuum spectrum of implantation abnormalities (24). A pregnancy may initially show CSP and then progress to deeper placental invasion during the second or third trimester. By the second trimester, all patients with CSP had most or all of the sonographic findings of MAP (24). All patients with CSP who had a live birth and hysterectomy were reported to have a histological diagnosis of MAP (24).
Management
There are currently no guidelines for optimal management of CSP. Most literature consists of case reports and small case series (3). Thirty-one different primary approaches to terminate a CSP have been reported, thus establishing the absence of any consensus (14). Management needs to be tailored to each patient and depends on the type of CSP, gestational age at diagnosis, whether implantation occurred into a scar or into a niche, patient’s age (1), hemodynamic stability (3), desire to preserve future fertility (1), presence of MAP (7), obstetrician’s comfort level, and availability of resources (3).
Nearly 50% of all CSP end in spontaneous abortion during the first trimester, oftentimes requiring surgery to control the bleeding (25). For hemodynamically stable patients with persistent type 1 CSP, options include termination or continuation of pregnancy (26). Termination during the first trimester of pregnancy is usually recommended (4). The objective is to remove the gestational sac, prevent complications, and maintain fertility by preserving the uterus (3).
Although medical therapy is less invasive, it is not suitable for patients with advanced gestation, hemodynamic instability, or uterine rupture (23). Surgical management may be considered in patients who fail medical treatment or become hemodynamically unstable (4).
Expectant management
There is currently no literature that supports expectant management (3), but some patients will make that choice. Until 2015, only 37 cases of CSP that continued under expectant management have been reported. From these cases, only 26 live births were reported between 26–39 weeks gestation with a hysterectomy performed in 27 of 36 patients (5). Thus, only 9 live births have been documented where a hysterectomy was avoided (5).
Patients who choose to continue pregnancy to viability with expectant management are followed by an obstetrician with frequent ultrasound scans at a centre with capability to perform a rapid Cesarean section and provide a massive transfusion protocol. Antenatal corticosteroids can be administered for fetal lung maturity (12). The goal is to reach near term or term (24). The likelihood of having an uneventful term pregnancy is low (16). Up to 50% of patients who chose expectant management experienced complications including cervical insufficiency, arteriovenous malformations, uterine rupture, severe hemorrhage, and DIC that required hysterectomy (22). Uterine rupture occurred in 32% of patients in all trimesters with the mean gestational age of 18.1 weeks (range, 6–28 weeks) (22). Uterine rupture in the first and second trimesters occurred in 21% of patients with CSP. Most cases of uterine rupture were painless but followed by acute bleeding, hemodynamic instability, hemorrhagic shock, and hemoperitoneum that required hysterectomy. Of those reported with uterine rupture as a result of CSP, 33% of patients required uterine artery embolization (UAE) (24). In a case series described by Timor-Tritsch et al., all pregnancies that progress into the second trimester developed MAP resulting in Cesarean hysterectomy with mean blood loss of 1,560 mL (range, 300–6,000 mL) (24).
Medical management
Reported medical management options include:
- Methotrexate
- Direct injection of other local embryocides, such as KCl, hyperosmolar glucose, crystalline trichosanthin with mifepristone. Case reports with high failure rates and severe hemorrhage requiring hysterectomy have been described (3);
- Direct injection of vasopressin into the gestational sac (13).
The most commonly used medical treatment option for CSP is systemic methotrexate (14). It can be used effectively in CSP with a gestational age less of than 8 weeks provided there is no fetal cardiac activity, and a beta-HCG <12,000 mIU/mL. Since methotrexate has a half-life of only 10 hours (12), multiple doses may be required (3). Complication rates of 62.1% have been reported (13). Due to limited absorption of systemic methotrexate by the fibrous scar tissue surrounding the gestational sac, direct injection into the gestational sac has been proposed for masses <3 cm in diameter and beta-HCG <20,000 mIU/mL. Direct injection carries a success rate of 61.1% (3). This can be performed transvaginally under ultrasound guidance (21). Local injection carries the risk of disrupting the uterine vascular supply, which can result in severe hemorrhage (2) that can present as late as 15 days after treatment (16). To minimize bleeding, placement of 18 French Foley or double-balloon catheter, intracervical vasopressin injection (16), and bilateral UAE have been separately described as adjuncts to local injection (2,3). Nonetheless, UAE can contribute to hypomenorrhea secondary to uterine and endometrial necrosis, and the impact of UAE on long-term fertility is unknown (3).
Medical management requires weekly follow-up of beta-HCG levels and monthly ultrasound evaluations until products of conception are no longer visualized (13). The time required for serum beta-HCG to reach undetectable levels is 21–188 days (16).
Surgical management
Reported surgical management options include:
- Hysteroscopy (13)
- Hysteroscopic removal of gestational tissue (13);
- Hysteroscopic hysterotomy (13);
- Hysteroscopic local methotrexate or ethanol injection (13);
- Hysteroscopic aspiration of gestational sac after local methotrexate injection (13);
- Hysteroscopy followed by laparoscopy/laparotomy to remove the ectopic mass (13);
- Hysteroscopy and systemic methotrexate (13);
- Hysteroscopy and vasopressin (13);
- Hysteroscopy and mifepristone (13);
- Hysteroscopy with transabdominal sonographic guidance (13).
- Laparoscopy or laparotomy with wedge excision of the ectopic mass and subsequent repair of myometrium (2). Repair of the uterine defect may be performed at the same time (4). Bilateral UAE prior to excision and vasopressin may be used to minimize bleeding (3);
- Ultrasound-guided gestational sac needle aspiration (13);
- Ultrasound-guided local intra-gestational methotrexate or KCl injection (13);
- Systemic methotrexate with ultrasound guided intra-gestational KCl injection (13);
- D&C (24)
- Transvaginal resection of CSP (13);
- Robotic-assisted laparoscopic removal of ectopic mass (3);
- UAE
- Bilateral hypogastric artery ligation (13);
- Transrectal ultrasound guided aspiration (13);
- Hysterectomy (23).
Although D&C is the most commonly reported surgical procedure, it has the highest rate of complications (62%) which include severe bleeding requiring hysterectomy. For this reason, several authors have recommended avoiding D&C (14). To minimize bleeding, the placement of a Foley catheter (13), Shirodkar cervical suture to occlude the cervix (27), or UAE have been performed (13).
Other commonly used surgical options are hysteroscopic excision and intra-gestational methotrexate with UAE (14). Ultrasound-guided intra-gestational methotrexate injection ± systemic methotrexate and surgical excision by hysteroscopic guidance had the lowest complication rate (14).
Future pregnancy
For patients who retain fertility, counselling regarding the risks in subsequent pregnancy is essential. There is no particular reason why implantation will be on the scar again. The current literature reports 64 pregnancies following CSP (14). Some authors recommend avoiding pregnancy for 12–24 months (18). There is insufficient evidence for whether a surgical repair of the uterine scar prior to pregnancy prevents CSP (14). It is important to have an early appointment with an obstetrician and perform an early ultrasound to confirm intrauterine location of the new gestation (16). In a case series of 22 patients with CSP by Gao et al., reproductive outcomes included uncomplicated term pregnancy, miscarriage, recurrent CSP, and infertility. The rate of successful subsequent pregnancy was 87.5%, recurrence of CSP was 11.1%, and live birth rate was 62.5% (28). Future pregnancies also carry the risk of uterine rupture and MAP. The timing of uterine rupture is unpredictable (18). One case of uterine rupture at 38 weeks has been reported resulting in maternal and fetal death. Future pregnancies are delivered by elective Cesarean section (16).
Strengths and limitations of this case report
Strengths of this case study include a positive outcome for both mother and infant. Despite a high-risk pregnancy, both mother and infant were discharged from hospital in good condition. This indicated a strong interdisciplinary team and rapid response to the uterine dehiscence during labour, and presence of NICU services.
Limitations of this case report are that the information summarized here is not generalizable to all cases of CSP. Each center’s ability to manage both the pregnancy and delivery of a CSP will vary depending on their available resources. As well, there is a publication bias in that cases of CSP with poor outcomes may be less likely to be presented as a case report. This could lead to an unrealistic view of potential outcomes for patients.
Conclusions
The diagnosis and treatment of CSP can pose a challenge for obstetricians. Due to the increasing incidence of Cesarean section worldwide and increasing awareness of CSP, it can be expected for the overall incidence of CSP to continue to rise. Clinical suspicion, early diagnosis, and timely intervention are essential. Delayed diagnosis can result in catastrophic complications. Although successful live births with expectant management have been reported, the prognosis is poor and may be complicated by uterine rupture, MAP, and life-threatening hemorrhage requiring hysterectomy. There is a strong need for evidence-based guidelines to assist obstetricians with management of this potentially life-threatening condition.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE Reporting Checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-19-39/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-19-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fenerty S, Gupta S, Anoakar J, et al. Cesarean scar ectopic pregnancy. Appl Radiol 2017;46:20-1.
- Persadie RJ, Fortier A, Stopps R. Ectopic pregnancy in a Cesarean scar: A Case Report. J Obstet Gynaecol Can 2005;27:1102-6. [Crossref] [PubMed]
- Docheva N, Slutsky E, Borella N, et al. The Rising Triad of Caesarean Scar Pregnancy, Placenta Percreta, and Uterine Rupture: A Case Report and Comprehensive Review of the Literature. Case Rep Obstet Gynecol 2018;2018:1-6.
- Onwonga L, Thapa S, Ochin H, et al. Causes, assessment and management of Cesarean scar pregnancy. Int J Pregn Chi Birth 2017;2:119-24.
- Tamada S, Masuyama H, Maki J, et al. Successful pregnancy located in a uterine Cesarean scar: A case report. Case Rep Womens Health 2017;14:8-10. [Crossref] [PubMed]
- Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220-7. [Crossref] [PubMed]
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus: an unusual cause of postabortal haemorrhage. A case report. S Afr Med J 1978;53:142-3. [PubMed]
- Seow KM, Huang LW, Lin YH, et al. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004;23:247-53. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Denver R. C-section rates continue to increase while birth rates decline | CIHI [Internet]. Cihi.ca. 2018. Available online: https://www.cihi.ca/en/c-section-rates-continue-to-increase-while-birth-rates-decline
- Mercer BM, Miodovnik M, Thurnau GR, et al. A Randomized Controlled Trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA 1997;278:989-95. [Crossref] [PubMed]
- Roy MM, Radfar F. Management of a Viable Cesarean Scar Pregnancy: A Case Report. Oman Med J 2017;32:161-6. [Crossref] [PubMed]
- Pędraszewski P, Wlaźlak E, Panek W, et al. Cesarean scar pregnancy – a new challenge for obstetricians. J Ultrason 2018;18:56-62. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of Cesarean deliveries: early placenta accreta and Cesarean scar pregnancy. A review. Am J Obstet Gynecol 2012;207:14-29. [Crossref] [PubMed]
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a Cesarean scar. Ultrasound Obstet Gynecol 2000;16:592-3. [Crossref] [PubMed]
- Ash A, Smith A, Maxwell D. Cesarean scar pregnancy. BJOG 2007;114:253-63. [Crossref] [PubMed]
- Nezhat C, Grace L, Soliemannjad R, et al. Cesarean scar defect: What is it and how should it be treated? OBG Management 2016;28:32-4.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006;107:1373-81. [Crossref] [PubMed]
- Wu R, Gupta M, Katz D, et al. Magnetic Resonance Imaging as an Adjunct to Ultrasound in Evaluating Cesarean Scar Ectopic Pregnancy. J Clin Imaging Sci 2013;3:16. [Crossref] [PubMed]
- D'Antonio F, Palacios-Jaraquemada J, Lim P, et al. Counseling in fetal medicine: evidence-based answers to clinical questions on morbidly adherent placenta. Ultrasound Obstet Gynecol 2016;47:290-301. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A, Santos R, et al. The diagnosis, treatment, and follow-up of Cesarean scar pregnancy. Am J Obstet Gynecol 2012;207:44.e1-44.e13. [Crossref] [PubMed]
- Timor-Tritsch IE, Khatib N, Monteagudo A, et al. Cesarean scar pregnancies: experience of 60 cases. J Ultrasound Med 2015;34:601-10. [Crossref] [PubMed]
- Birch Petersen K, Hoffmann E, Rifbjerg Larsen C, et al. Caesarean scar pregnancy: a systematic review of treatment studies. Fertil Steril 2016;105:958-67. [Crossref] [PubMed]
- Timor-Tritsch IE, Monteagudo A, Cali G, et al. Caesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol 2014;44:346-53. [Crossref] [PubMed]
- Jurkovic D, Knez J, Appiah A, et al. Surgical treatment of Caesarean scar ectopic pregnancy: efficacy and safety of ultrasound-guided suction curettage. Ultrasound Obstet Gynecol 2016;47:511-7. [Crossref] [PubMed]
- Timor-Tritsch I, Monteagudo A, Agten A. Caesarean scar pregnancy diagnosis and management. Contemp Obstet Gynec 2015;34:601-10.
- Jurovic D, Ben-Nagi J, Ofilli-Yebovi D, et al. Efficacy of Shirodkar cervical suture in securing hemostasis following surgical evacuation of Cesarean scar ectopic pregnancy. Ultrasound Obstet Gynecol 2007;30:95-100. [Crossref] [PubMed]
- Gao L, Huang Z, Zhang X, et al. Reproductive outcomes following Caesarean scar pregnancy – a case series and review of the literature. Eur J Obstet Gynecol Reprod Biol 2016;200:102-7. [Crossref] [PubMed]
Cite this article as: Giroux M, Kamencic H, Fras T, McLellan S, Onasanya O, Adanlawo A, Patel R, Carson G. Expectant management of a viable Cesarean scar pregnancy complicated by uterine dehiscence and massive hemorrhage: a case report and literature review. Gynecol Pelvic Med 2021;4:9.



