
Anatomical and functional outcomes of robotic abdominal lateral suspension for high-stage anterior-apical prolapse: a case series of 93 patients
Introduction
The abdominal lateral suspension (ALS) using a T-shaped prosthetic device could be considered an alternative approach to repair advanced apical prolapse avoiding challenging management of the sacral promontory area.
Kapandji in 1967, as well as Cornier and Madelenat in 1994 first, describe this innovative surgical operation (1,2). The surgery has then been adapted and modified to the traditional laparoscopic approach by Dubuisson in the 90s’ (3-5). The laparoscopic lateral suspension (LLS) has been identified as a safe technique concerning the combined outcome, negligible adverse events, and high long-term subjective cure rates relative to the restoration of apical and anterior advanced prolapse avoiding the risky steps during LSC approach to the presacral area. Data on literature about lateral suspension performed by the traditional laparoscopic approach (LLS) describe an objective cure rate of >90% after 1 year on both the anterior and apical prolapse that is similar to that observed for laparoscopic sacrocolpopexy (LSC) (3-8).
This minimally invasive operation may also be performed using robotic multiport or single-port platforms (9-12). Nowadays, few publications about the robotic-assisted lateral suspension (RALS) are present in the scientific literature and these studies are designed as a retrospective and no prospective trials (9,10). The first robotic series was reported in 2014 by Dällenbach, who described the feasibility of this novel technique using a robotic platform in a small series of ten symptomatic women with concomitant anterior and apical prolapse (12). In 2016, our group described the technical features and the short-term outcomes of the initial forty cases of RALS, reporting an objective cure rate of more than 85% for the anterior and more than 90% for the apical compartment; moreover, the subjective cure rate was near 80% (10). Here we described surgical and anatomical medium-term outcomes of a series of 93 RALS for the restoration of concomitant advanced anterior-apical prolapse.
We present the following article in accordance with the STROBE reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-30/rc).
Methods
Using a retrospective study design, we performed a Cohort study evaluating symptomatic Caucasian women with primary or recurrent stage III/IV anterior and apical prolapse underwent RALS between September 2014 and September 2018.
Patients sample and pre- and post-operative clinical assessment
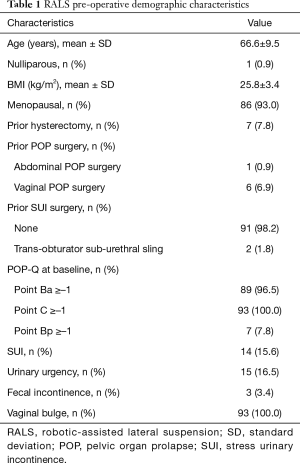
Data about the first ninety-three consecutive minimally invasive lateral suspension with the robotic approach for symptomatic high (3rd/4th) stage concomitant apical and anterior prolapse and no (n=86) or minimal (stage 1st, n=7) posterior compartment prolapse were evaluated. Baseline characteristics, surgical history and pelvic organ prolapse (POP)-related symptoms of patients are described in Table 1. All patients enrolled in the study claimed POP-related symptoms. The vaginal bulge and obstructed voiding were the most bothersome POP symptoms claimed at the pre-surgical assessment.
Full table
The most significant symptoms were vaginal bulging, voiding impairment, and urgency symptoms (Table 2). Considering that in the study population there was no case of current or latent stress urinary incontinence (SUI), no surgical procedure for SUI was made. The surgical procedure was analogous in all enrolled patients and was executed by a single operator with advanced skills in minimally invasive techniques for pelvic surgery (TS). All patients underwent surgery at the Multidisciplinary Center of Robotic Surgery of Cisanello University Hospital of Pisa. The da Vinci Si and Xi systems (Intuitive Surgical®, Sunnyvale, CA, USA) were used for surgeries. All women enrolled for surgery were informed about the benefits and the risks and different surgical options and signed informed consent in accordance with the Declaration of Helsinki. The Institutional Review Board of the University of Pisa approvals was obtained for this study (protocol approval: 808/2015). The present study was carried out in accordance with the recommendations of the Good Clinical Practice (ICH/GCP), Ministerial Decree of 1997. All patients enrolled for the study were previously evaluated during the pelvic floor disease consultation activity of the Santa Chiara University Hospital in Pisa. All patients underwent pre-surgical assessment comprised of medical history, physical, pelvic ultrasound and urogynecological examination, and laboratory exams. Data of urogynecological evaluation and questionnaires regarding functional outcomes were collected at the time of surgery, at the time of discharge and 1 month later during the first post-operative clinical check-up. No systematic pre-operative urodynamic investigation was executed. The subjective cure rate of the most bothersome symptom was reported on the evaluation of the patients using a visual analog scale (VAS) ranging from 0 to 10 (0: no improvement, 10: 100% improvement). The Incontinence Impact Questionnaire 7 (IIQ7) was employed to evaluate the effect of UI in four domains (physical activity, social relationships, travel, and emotional health) using a 4-point response scale (0= not at all to 3= greatly) with a total score range from 0 to 28 (13). The quality of life (QOL) related to prolapse disease was measured by the validated P-QOL questionnaire assessment (14). The defecatory function was examined by the “Wexner constipation” score (15).

Full table
Patients’ charts were examined for the determination of the complications that occurred during surgery, perioperatively and during the early post-operative periods. Complications were classified according to the “Clavien-Dindo classification of surgical complications scale” (16).
The step by step robotic technique is described according to the procedure defined by Simoncini et al. (10). A video of the surgical procedure is available as an adjunctive material (Video 1).
Description of the surgical procedure
Patients positioning, trocars placement and docking of the robot
The first step of the procedure is to grasp the cervix using two tenacula that are vertically positioned on the left and right sides of the external uterine orifice. The insertion of an hysterometer in the uterine cavity allows the assistant to manipulate the uterine body during the surgical procedure. Once introduced a 12-mm optic trocar and the Da Vinci 30° optic in the umbilical scar area, the assistant positioned a robotic 8-mm trocar in the right and left iliac fossa about 10 cm laterally and 2 cm caudally to the umbilical trocar under direct vision. Moreover, a 12-mm trocar is placed in the left upper abdominal quadrant for the assistant operator. After a Trendelenburg sequence, the patient is finally placed in a 20° Trendelenburg position, and the robotic platform is docked from the left side of the patient.
Vesical-vaginal space dissection
The dissection of the vesical-vaginal space is facilitated by the assistant pushing and slightly lifting the uterine cervix retroverting the uterine body in a middle position.
The assistant moves a malleable straight retractor in the vagina to facilitate the exposure of the anterior vaginal wall and fornix during the identification and the dissection of the vesical-vaginal septum. The assistant must progressively determine intensifications in the cervix’s thrust and on the vaginal retractor for achieving a deep exposure of the vesical-vaginal septum up to the bladder trigone.
Introduction, placement and suture of the anterior arm of the mesh
In order to enable insertion through the 12-mm left paraumbilical port and intracorporeal mesh maneuvering, the two lateral arms of a titan-covered T-shaped polypropylene mesh (TiLOOP® “Prof. Dubuisson”® 9×41.5 cm, 65 g/m2) are previously rolled and stitched. The 6 cm length and 5 cm width anterior arm of the mesh is modelled over the anterior vaginal wall under the guidance of the vaginal retractor and stitched to the anterior vaginal wall using six stitches of 2-0 long-term absorbable synthetic monofilament suture of Glycolide and Trimethylene carbonate (Maxon®, Covidien). A third apical row of three non-absorbable 2-0 polypropylene sutures (Prolene®, Ethicon) is then placed on the anterior and right and left sides of the cervix, and the last polypropylene suture was used to fix the T-shaped graft to the isthmus.
Abdominal access and mesh retraction
After the remotion of the blocking sutures, the mesh’s long lateral arms are freed in the abdomen. The skin is incised 3-mm in a peculiar position located 2 cm above and 2 cm laterally to the anterior superior iliac spine, bilaterally. The operator or the assistant inserts in the skin incisions a laparoscopic dissector and pushes orthogonally through the fascia to develop a retroperitoneal space up to the pelvis. After that, the laparoscopic tool is turned towards the center of the pelvis to create a retroperitoneal tunnel up to the round ligament to reach the lateral arm of the mesh that is then taken and laterally pulled out gently by retracting the laparoscopic grasper up to the abdominal skin incision. The operator must take care not to damage external iliac vessels bilaterally during the introduction and retraction of the laparoscopic tool. The lateral arms of the mesh are not routinely sutured to the fascia according to the “tension-free” repair principle in the first series of 40 patients and the last series of 36 cases, while in 17 cases, a suture of the lateral mesh to the abdominal wall was performed using a 2-0 glyconate suture (Monosyn®, B-Braun Melsungen AG) bilaterally. Before the closure of the skin incisions, the lateral arms of the mesh are cut at the level of the skin surface.
Closure of the pre-vesical peritoneum and undocking of the robot
The pre-vesical peritoneum is sutured over the mesh to cover the prosthesis with a continuous 2-0 glyconate suture (Monosyn®, B-Braun Melsungen AG) at the end of the surgery.
Statistical analysis
Continuous variables were described as means ± standard deviation (SD), whereas categorical variables were presented as percentages. Shapiro-Wilk normality test was used to assess the normality of data distribution. Wilcoxon signed-rank and Mann-Whitney tests were used to compare pre- with post-surgical parameters. Kruskal-Wallis test was performed, followed by Dunn’s multiple comparisons test to evaluate the outcomes among post-operative evaluations at 0, 6, 12 and 24 months. A P<0.05 value was considered statistically significant. All statistical analyses were performed using SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA).
Results
Perioperative, anatomical and functional outcomes
All the cases of RALS were completed by a full robotic approach with no conversion to open or traditional laparoscopic approach and no intraoperative adverse event. The mean operating time resulted 129±34 min, the mean blood loss was 20 mL, and the mean days of post-operative hospitalization resulted in one day. Only three patients underwent supracervical hysterectomy for fibromatosis conditions at the surgery time, while the uterus was preserved in 83 patients. Seven women with a previous history of hysterectomy performed RALS on the vaginal vault. Twelve patients underwent concomitant surgeries during RALS: stapled transanal rectal resection (STARR) (n=1), cervical amputation (n=1), ovarian cyst removal (n=1) and bilateral adnexectomy (n=9).
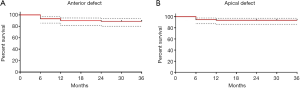
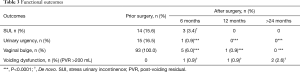
The mean post-operative follow-up was 26±6 months. We described significant restoration in the POP-Q score in all repaired compartments with an overall objective cure rate for the anterior and apical compartments of 88.8% and 93.6%, respectively [confidence interval (CI): 3.65–8.64 for anterior and CI: 3.10–7.35 for apical compartment].
Anatomical outcomes are shown in Figure 1A,B and Tables 2,3.
Full table
An anatomical relapse was observed in 17 cases (18%), and the re-intervention was necessary for 13 patients (14%). Most relapses were reported within the first 6 months after surgical treatment, as described in Table 3. Six patients (6.9%) claimed relapse of isolated prolapse involving the bladder and/or the anterior vaginal wall. In particular, all these patients presented recurrence of cystocele that resulted caudal to the mesh’s anterior flap, which otherwise continued to be adequately suspended and secured to the anterior vaginal wall and the cervical and isthmic portion of the uterus. All these women presenting symptomatic relapse of the anterior compartment were scheduled for anterior colporrhaphy that resulted appropriate in the restoration of the new anatomical defects.
Four patients (4.3%) presented symptoms of concomitant apical and anterior anatomical failure due to the sliding of the lateral arms. All these patients needed an abdominal re-intervention that was repeated using both traditional laparoscopic and robotic approaches. In detail, in two cases, a laparoscopic retraction of the lateral arms of the mesh was feasible, while a colpo/cervicosacropexy was performed after removal of the lateral arms of the mesh in three cases. None of these cases which underwent re-intervention showed further recurrence. Three patients had an isolated and asymptomatic IInd stage apical prolapse, not requiring a re-intervention. Remarkably, two patients developed an asymptomatic cervical elongation at post-operative follow-up, but we did not know pre-surgical cervical length, so that it is unclear whether the elongation was present before surgery. At enrollment, 7 patients (7.8%) had a minimal high rectocele (POP-Q stage I), which was resolved after treatment in five cases and persisted in the other two cases. In the rest of the study population, no posterior compartment prolapse was described.
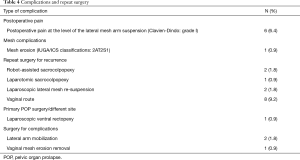
However, 5 (5%) patients developed an asymptomatic de novo moderate-high rectocele (POP-Q stage I–II). The overall rate of re-surgery is described in Table 4. Fourteen patients (15.6%) had occult SUI at a pre-surgical clinical assessment. These patients continued to be continent after surgical treatment. De novo SUI occurred in three cases (3.4%), and a mid-urethral sling was positioned in two patients. Fifteen patients (16.5%) had urinary urgency without incontinence before the operation. In 14 cases, the urgency was resolved after surgical treatment, probably thanks to the reparation of the anterior prolapse. No patient developed de novo urge symptoms after RALS. Voiding dysfunction within 1 year in the absence of a prolapse relapse was described in two patients and was associated with a post-voiding residual (PVR) >150 mL. Obstructed defecation symptoms after surgery were experienced by one patient but no case of post-operative fecal incontinence or dyspareunia were described. In terms of the absence of a perceived vaginal bulge, the subjective cure rate was 88.8% at 2 years’ follow-up. Six patients claimed post-operative pain at the lateral mesh arm site retraction (Clavien-Dindo I). In two cases, the surgical mobilization of the graft was necessary considering that the fascial pain was reported in the area where the mesh reached the abdominal wall, even if the lateral arms of the mesh have been fixed to the abdominal fascia with stitches in only 10% of cases. The surgical treatment consisted of a small skin incision, mesh identification and isolation and the detachment of the lateral arm of the mesh from the fixation point on the fascia with subsequent prompt resolution of pain symptoms.
Full table
However, two patients experienced pain due to a mono-lateral hematoma, while the other four patients had spontaneous pain relief 3 months after surgical treatment.
One patient experienced anterior vaginal wall mesh exposure that occurred within 2 months of surgery. The vaginal exposure was grade 1 (<1 cm) and was placed away from the suture lines, classified as 2AT2S1, according to the IUGA/ICS Prosthesis/Graft Complication Classification System. This complication is needed for vaginal revision with partial mesh excision. No patient had significant post-surgical complications (Clavien-Dindo grade ≥ 3a).
Assessment of patient satisfaction
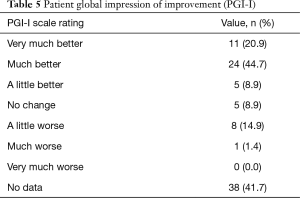
At 18–24 months post-operation follow-up, 55 women (58.3% of the study population) participated in an interview. There are 65.6% of participants considered themselves “much better or better” than before surgical treatment according to the PGI-I scale. The report of telephone interviews is described in Table 5.
Full table
Discussion
Our results confirm previous experiences with a peculiar surgical technique to perform RALS, thus suggesting that this procedure is feasible, highly reproducible and safe, and it is effective in the treatment of high-stage concomitant anterior and apical pelvic prolapse (10,12). The results of our case series of patients treated by RALS using a T-shaped titanized mesh placed in the vesicovaginal space demonstrated that this procedure effectively restores high-grade concomitant apical and anterior POP with the total improvement of prolapse symptoms and significant subjective patients’ satisfaction rates. These findings mirror the results achieved by Dubuisson et al. using the traditional laparoscopic technique (3-5,7,8,17); however, our results derived from a sample of patients with more advanced prolapse (stage III/IV), with respect to those enrolled by Dubuisson and colleagues. According to the anatomical outcomes described in our case series, we can affirm that minimally invasive lateral suspension of the apical compartment may be effective both for the restoration of moderate and advanced anterior/apical prolapse (5,7,8,10,12).
We believe that RALS is not the gold standard procedure for treating patients with concomitant advanced apical/posterior prolapse.
Considering that the axis of the apical traction determined by the lateral arms of the mesh could not result in an ideal closure of the Douglas pouch, thus determining the progression of the posterior compartment prolapse; we think that in these cases, patients selected for RALS should also undergo associated trans-vaginal or trans-rectal posterior surgery.
The outcomes derived from our current and earlier series demonstrated that RALS is a well tolerable intervention that results in a successful reparation of anterior and apical anatomical supports while preserving the natural orientation and length of the vagina, which is relevant for sexual activity. In our opinion, the robotic platform allows dissecting the vesicovaginal plane accurately deeply to the retro-trigonal area. This surgical task is critical to achieving the mesh’s accurate placement in a position that may permit complete restoration of the anterior anatomical defect. Furthermore, it is plausible to assume that robotic tools may allow for faster and fluid placement of sutures, resulting in a stable fixation of the mesh to the anterior vaginal wall and the apex. From a technical standpoint, suturing with the robotic assistance allows simpler and more relaxing stitches positioning respect to standard laparoscopic technique; however, it has not been demonstrated that this technical advantage necessarily translates into more excellent durability and better quality of anatomical correction; therefore, data of the literature are still insufficient and not conclusive (18,19).
Parallel, it is not yet clear whether the execution of this surgery with robotic assistance can reduce the incidence of surgical complications related to the prosthesis hypothesizing the possibility of an accurate and deep dissection of the vesicovaginal space as well as the fluid placement of stitches on the mesh with optimization of the tension on a large vaginal surface thus minimizing wrinkling.
We hypothesize that RALS may be a convenient alternative to abdominal sacrocolpopexy (ASC) in several disorders such as pelvic anatomic aberrations that make mesh fixation to the longitudinal ligament on the presacral area difficult or when promontory isolation and dissection are complicated in the presence of a fatty pre-sacral space. Other pros of RALS over abdominal sacral suspension if the apex could include reducing the risk of nerves and vessels damages and sacral vertebral osteomyelitis or intervertebral discitis related to stitches positioning on the presacral area.
Moreover, RALS may be a rescue surgery to treat patients with apical relapse previously undergone ASC, thus escaping the need of performing new surgical procedures in anatomical spaces where the previous graft was positioned. In this view, RALS could present some advantages over ASC in perspective; however, literature in this field is still lacking. The principal limit of the present study is the retrospective design of the report.
Concluding, this report describes outcomes a safe, effective and reproducible procedure that successfully restores concomitant anterior and apical prolapse.
For patients with concomitant posterior and apical POP, we still consider a supracervical hysterectomy with the suspension of the cervix to the sacrum, the gold standard procedure, because we believe that placing a mesh reinforcement in the recto-vaginal space and determining the posterior direction of the vaginal axis will result in an adequate anatomic restoration of the apical-posterior defect.
New trials with a comparative design with ASC procedure and with longer follow-up are mandatory to investigate the value and the putative role of this novel robotic POP surgery within the huge number of surgical options used for the treatment of advanced apical prolapse.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-30/rc
Data Sharing Statement: Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-30/dss
Peer Review File: Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-30/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-30/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the University of Pisa approvals was obtained for this study (Protocol approval: 808/2015) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kapandji M. Treatment of urogenital prolapse by colpo-isthmo-cystopexy with transverse strip and crossed, multiple layer, ligamento-peritoneal douglasorrhaphy. Ann Chir 1967;21:321-8. [PubMed]
- Cornier E, Madelenat P. The M. Kapandji hysteropexy: a laparoscopic technic and preliminary results. J Gynecol Obstet Biol Reprod (Paris) 1994;23:378-85. [PubMed]
- Dubuisson JB, Eperon I, Jacob S, et al. Laparoscopic repair of pelvic organ prolapse by lateral suspension with mesh: a continuous series of 218 patients. Gynecol Obstet Fertil 2011;39:127-31. [Crossref] [PubMed]
- Dubuisson JB, Yaron M, Wenger JM, et al. Treatment of genital prolapse by laparoscopic lateral suspension using mesh: a series of 73 patients. J Minim Invasive Gynecol 2008;15:49-55. [Crossref] [PubMed]
- Eperon I, Luyet C, Yaron M, et al. Laparoscopic management of genital prolapse by lateral suspension using mesh: a series of 377 patients. Rev Med Suisse 2011;7:2084-2086-8. [PubMed]
- Dubuisson JB, Dubuisson J. How I do. laparoscopic repair of vaginal vault prolapse by lateral suspension. Gynecol Obstet Fertil 2012;40:617-9. [Crossref] [PubMed]
- Veit-Rubin N, Dubuisson JB, Gayet-Ageron A, et al. Patient satisfaction after laparoscopic lateral suspension with mesh for pelvic organ prolapse: outcome report of a continuous series of 417 patients. Int Urogynecol J 2017;28:1685-93. [Crossref] [PubMed]
- Veit-Rubin N, Dubuisson JB, Lange S, et al. Uterus-preserving laparoscopic lateral suspension with mesh for pelvic organ prolapse: a patient-centred outcome report and video of a continuous series of 245 patients. Int Urogynecol J 2016;27:491-3. [Crossref] [PubMed]
- Dällenbach P, Petignat P, Dubuisson JB, et al. LESS, NOTES and robotic surgery in gynecology: an update and upcoming perspectives. Rev Med Suisse 2010;6:2024-2026-9. [PubMed]
- Simoncini T, Russo E, Mannella P, et al. Robotic-assisted apical lateral suspension for advanced pelvic organ prolapse: surgical technique and perioperative outcomes. Surg Endosc 2016;30:5647-55. [Crossref] [PubMed]
- Giannini A, Russo E, Mannella P, et al. Single site robotic-assisted apical lateral suspension (SS R-ALS) for advanced pelvic organ prolapse: first case reported. J Robot Surg 2017;11:259-62. [Crossref] [PubMed]
- Dällenbach P, Veit N. Robotically assisted laparoscopic repair of anterior vaginal wall and uterine prolapse by lateral suspension with mesh: initial experience and video. Int Urogynecol J 2014;25:1137-9. [Crossref] [PubMed]
- Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Qual Life Res 1994;3:291-306. [Crossref] [PubMed]
- Lenz F, Stammer H, Brocker K, et al. Validation of a German version of the P-QOL Questionnaire. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:641-9. [Crossref] [PubMed]
- Agachan F, Chen T, Pfeifer J, et al. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 1996;39:681-5. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Dubuisson JB, Jacob S, Chapron C, et al. Laparoscopic treatment of genital prolapse: lateral utero-vaginal suspension with 2 meshes. Results of a series of 47 patients. Gynecol Obstet Fertil 2002;30:114-20. [Crossref] [PubMed]
- Paraiso MF, Jelovsek JE, Frick A, et al. Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol 2011;118:1005-13. [Crossref] [PubMed]
- Stefanidis D, Hope WW, Scott DJ. Robotic suturing on the FLS model possesses construct validity, is less physically demanding, and is favored by more surgeons compared with laparoscopy. Surg Endosc 2011;25:2141-6. [Crossref] [PubMed]
Cite this article as: Giannini A, Russo E, Misasi G, Montt-Guevara MM, Mannella P, Simoncini T. Anatomical and functional outcomes of robotic abdominal lateral suspension for high-stage anterior-apical prolapse: a case series of 93 patients. Gynecol Pelvic Med 2021;4:3.


