
Current status on treatment of uterine adenosarcoma: updated literature review
Introduction
Uterine adenosarcoma (UAS) is a rare female genital tract malignancy accounting for 5–9% of uterine sarcomas and only about 0.2% of all uterine neoplasm (1,2).
It is considered a biphasic tumor because of the presence of benign epithelial elements combined with a malignant mesenchymal component (3,4). Since that the latter is typically low-grade, adenosarcoma is considered lesser aggressive than its epithelial counterpart, uterine carcinosarcoma.
Unfortunately, given the extreme rarity of this subtype of neoplasm, there are limited data in literature to help decision making in clinical practice, and the most data we have available derived from retrospective case series and shared clinical experiences.
Herein we present an updated literature review on the current management of UAS, focusing on the role of not only medical but also surgical treatment, in order to improve outcomes of patients affected by this extremely rare disease.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-81/rc).
Methods
Information used to write this paper was collected from the sources listed in Table 1.

Full table
Discussion
Clinical features
Adenosarcoma mostly involves uterus, but it can arise from other tissue such as ovaries, cervix, vagina, fallopian tubes, and pelvis particularly in a contest of endometriosis (5-9). More rarely adenosarcoma can also involve extrapelvic sites (10-18).
Although it has been initially described as an advanced age tumor, the pick of incidence is around the fifth and sixth decades, with no significant difference in ethnicity (1,19,20).
Among the risk factors, endometriosis seems to play an important role, although a certain connection is not yet established. In a group of 1,000 patients with proved endometriosis, a cancer incidence of 5.5%, particularly endometroid carcinoma, but also clear cell carcinoma and adenosarcoma have been reported (21). A molecular mechanism leading to this malignant transformation is not completely understood. A repeated DNA damage from oxidative stress caused by menstruation, and consequential iron overload could be involved (22).
Previous pelvic irradiation and treatment with tamoxifen or other estrogen-modulating agents, maybe due to endometrial partial estrogen agonist effect, may represent an additional risk factor of adenosarcoma (23-33).
Since that UAS usually present as a polypoid mass within the uterine cavity, the most common clinical presentation is an abnormal uterine bleeding, observed in about 65–76% (3,23,34).
Other symptoms are pelvic pain or pelvic mass, vaginal discharge, abdominal discomfort and distention, especially for ovarian adenosarcoma, that can reach large size, even up to 50 cm (35).
According to 2009 FIGO staging, which represents the current staging system for adenosarcoma, the presence of myometrial invasion as well as the extent of disease outside the uterus determine the stage of disease (36).
The incidence of local and distant recurrences over a period of about 10 years varies from 14.3% to 45% and it is higher in patients with the presence of sarcomatous overgrowth (23,37).
It generally recurs locally, such as for ovarian adenosarcoma, but also with distant metastases (especially lung and liver).
The 5-year overall survival (OS) is about 50–60% for patients with adenosarcoma with myometrial invasion or/and sarcomatous overgrowth compared to 70–80% for patients with early stage adenosarcoma (4).
Pathologic and molecular features
Microscopically, adenosarcoma is represented by a benign glandular epithelial component and a malignant mesenchymal component, characterized by spindled cells surrounding glands in the form of peri-glandular cuffs characterized by cellular atypia and high mitotic activity (2,38).
A mitotic rate (at least 2 per 10 high-power fields) is necessary to make the diagnosis of adenosarcoma according to the WHO criteria. Ki67 index is usually under 5%, increasing to 20% in peri-glandular cuffs.
Necrosis, myometrial and lymphovascular invasion have been observed in 35%, 16–74% and 9–16% cases respectively (37).
The presence of a sarcomatous overgrowth, intended as more than 25% of the tumor composed of pure sarcoma, is the most important histological prognostic factors in adenosarcoma and it is directly related to the grade of disease (3,23,39).
Even if not pathognomonic, the most common immunohistochemical staining in adenosarcoma are CD10 (7–100%) and WT1 (79%) similar to endometrial stromal tumors. Additional markers could be vimentin (86%), smooth muscle actin (50–68%), desmin (32–62.5%), CD34 (35%), calretinin (12%) and AE1/3 cytokeratin (25–27%); more rarely focal positivity for inhibin and c-kit have been observed (40).
The epithelial component usually presents a positive staining for cytokeratins, EMA, estrogen receptor (ER) and progesterone receptor (PR).
Generally, the immunohistochemical staining for ER and PR varies from 15% to 95% and the loss of ER and PR expression has been associated with sarcomatous overgrowth (40). However, prognostic value of ER and PR expression in UAS has still to be defined.
The overexpression of PDGFR-α and expression of β-catenin have been found in most of adenosarcoma (7,41).
Few studies on molecular biology of adenosarcomas are available. Currently no differences in mutational profile between adenosarcoma with or without overgrowth have been described, even if the cases with sarcomatous overgrowth presented an higher copy number variations (42,43).
MDM2 and CDK4 genes amplification as well as alterations in the PIK3CA/AKT/PTEN pathway have been commonly observed. TP53 mutation, even if rare, are often related to sarcomatous overgrowth and consequently associated with aggressive clinical behavior (43).
Treatment
Surgery
The standard of care for localized UAS is total hysterectomy (2,38,44). Local excision could be reserved in case of desire of fertility preservation in very selected reproductive-age women without myometrial invasion and without sarcomatous overgrowth tumors (45).
Because of the incidence of the local tumor spread to the adnexa and ovaries (17% and 8% respectively), as well as the known frequent expression of ER and PR, bilateral salpingo-oophorectomy (BSO) is strongly recommended (3,46,47).
There is no evidence in favor of lymphadenectomy in addition to hysterectomy, since that the incidence of lymph node metastases in adenosarcoma is around 2.9% and the impact of lymphadenectomy upon survival is still unclear (48).
About surgery and HIPEC only two cases have been reported in literature, showing little benefit as a salvage therapy (49).
Long-term follow-up of the patients is recommended giving the risk of late recurrence over a period of about 10 years.
Adjuvant radiation therapy
Although the National Compressive Cancer Network (NCCN) uterine cancer guidelines recommend adjuvant radiation in patients with stage II to IVa high-grade endometrial stromal sarcoma, there are no data about the efficacy of radiation therapy in UAS (46).
Neo/adjuvant chemotherapy
The role of neoadjuvant and adjuvant chemotherapy in this disease is still controversial. Given the rarity, adenosarcoma cases have been often included in trials with other uterine mesenchymal malignancies, therefore clinical trials on adjuvant treatment for adenosarcoma only lack.
Currently, the benefit of adjuvant chemotherapy on progression-free survival for adenosarcoma is only documented in some case reports using different regimens (3,50-62) (Table 2).
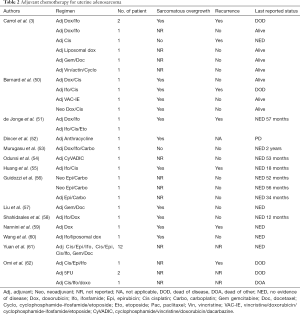
Full table
Although adjuvant therapy is generally not recommended, it could be considered in patients with high risk of recurrence, due to presence of myometrial invasion or/and sarcomatous overgrowth, by a shared decision-making process. Conversely, for patients at low risk of disease relapse, the strategy of choice should be observation alone (63).
Hormonal therapy
The ER/PR positivity could be used as predictive biomarkers for response to hormonal therapy, but currently, the evidence is limited to only case and series reports both in adjuvant and the recurrent/metastatic setting. In the latter, responses between 10 months to 7 years have been reported (3,64-67).
Given the generally lack of ER and PR expression in the most of high-grade/sarcomatous overgrowth disease, hormonal therapy should be considered only in low grade ER/PR positive adenosarcoma without sarcomatous overgrowth (68).
The most used agents include synthetic progesterones (megestrol acetate, medroxyprogesterone, dienogest), GnRH agonists (leuprolide), aromatase inhibitors (anastrazole, letrozole) and SERMs (tamoxifen, raloxifen).
Nathenson et al. described a stable disease and an improved survival for 4 patients treated with GnRh agonist and aromatase inhibitors for 2 to 15 years but these data are insufficient to determine the benefit of hormonal therapy in a larger population (69).
Treatment for advanced or metastatic disease
In the most cases adenosarcoma tends to recur locally, within the pelvis and abdominal cavity. Two larger case series showed a local recurrence in 22% of 74 patients to 42% in 100 patients respectively (3,23). Distant metastases, mainly localized in the lung and liver, but also involving bone, kidney, spleen and more rarely the brain, are less common.
Although there is no a standard approach for recurrent/metastatic disease, the management of advanced/metastatic adenosarcoma is generally medical (chemotherapy and hormonal therapy), even if surgery could be an option when feasible in selected cases. Radiation has a role of palliation in symptomatic cases.
The role of secondary surgery has been supported by two case series: a median OS of 58.4 vs. 30.1 months (HR 0.68), in the 62% of 32 recurrent patients underwent surgical cytoreduction have been shown (3); moreover, an increased time to second recurrence for patients who undergo a second surgery (29.7 vs. 12.7 months) have been reported in a second series (47).
Therefore, in case of isolated local recurrence, surgical resection could be recommended.
Regarding chemotherapy, in case of locally or distant recurrences, case reports and case series described responses with the use of doxorubicin-based regimens (47,55,70-73), gemcitabine/docetaxel (34,47) and trabectedin (74), which generally are the standard treatments for uterine sarcomas (Table 3).
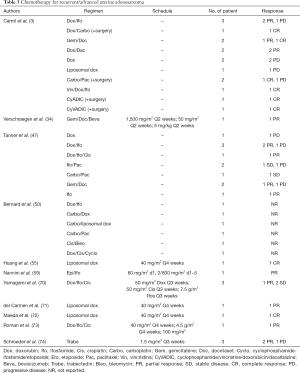
Full table
Nathenson et al. showed, in a large retrospective report about the use of systemic chemotherapy in recurrent UAS, a longer PFS (15.4 months) in patients treated with the association of doxorubicin and ifosfamide compared to doxorubicin alone or other regimens (69).
Doxorubicin/ifosfamide seems to have advantages also to reduce tumor size prior to surgery of recurrent disease. Whereas gemcitabine/docetaxel should be used in older patient or patient with comorbidities.
For rapid progression on previous anthracycline or gem/docetaxel regimens, Schroeder et al. published positive results about prolonged clinical benefit of three patients with relapsed UAS treated with trabectedin (74).
There are no prospective comparison studies about the efficacy and response of these regimens but there could be a benefit in OS by a sequential and integrated approach (surgery, hormonal therapy or further chemotherapy). Indeed, Nannini et al. reported a case report of a patient with advanced adenosarcoma at the time of diagnosis, treated with emergency simple hysterectomy, followed by chemotherapy with epirubicin/ifosfamide and, after an early radiologic response with marked shrinkage, underwent radical surgery with a complete response (59).
Conclusions
Adenosarcoma is a rare form of uterine sarcoma composed from a dual component (benign epithelial and malignant mesenchymal) mainly involving uterus but also other pelvic or peritoneal tissues.
Given the extreme rarity of this neoplasm, in clinical practice the management of adenosarcoma is still based on limited data and shared experiences of single centers data (Figures 1,2).
Additional data are needed to better understand the molecular background and clinical behaviors of this rare disease and improve patient outcomes with more tailored medical treatments.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Anna Myriam Perrone and Pierandrea De Iaco) for the series “Uterine Sarcomas” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-81/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.org/article/view/10.21037/gpm-20-81/coif). The series “Uterine Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nathenson MJ, Ravi V, Fleming N, et al. Uterine Adenosarcoma: a Review. Curr Oncol Rep 2016;18:68. [Crossref] [PubMed]
- D’Angelo E, Prat J. Uterine sarcomas: A review. Gynecologic Oncology. Gynecol Oncol 2010;116:131-9. [Crossref] [PubMed]
- Carroll A, Ramirez PT, Westin SN, et al. Uterine adenosarcoma: An analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol 2014;135:455-61. [Crossref] [PubMed]
- Krivak TC, Seidman JD, McBroom JW, et al. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: Comparison of treatment and survival. Gynecol Oncol 2001;83:89-94. [Crossref] [PubMed]
- Valdez VA, Planas AT, Lopez VF, et al. Adenosarcoma of uterus and ovary. A clinicopathologic study of two cases. Cancer 1979;43:1439-47. [Crossref] [PubMed]
- Chin PS, Chia YN, Lim YK, et al. Diagnosis and management of Müllerian adenosarcoma of the uterine cervix. Int J Gynaecol Obstet 2013;121:229-32. [Crossref] [PubMed]
- Gallardo A, Prat J. Mullerian adenosarcoma: A clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol 2009;33:278-88. [Crossref] [PubMed]
- Verschraegen CF, Vasuratna A, Edwards C, et al. Clinicopathologic analysis of mullerian adenosarcoma: The M.D. Anderson Cancer Center experience. Oncol Rep 1998;5:939-44. [Crossref] [PubMed]
- Clement PB, Scully RE. Extrauterine mesodermal (mullerian) adenosarcoma. A clinicopathologic analysis of five cases. Am J Clin Pathol 1978;69:276-83. [Crossref] [PubMed]
- Lazar RI, Straja T, Bratucu B. Uterine adenosarcoma metastasizing to the retroperitoneum. The impact of vascular involvement. J Med Life 2012;5:145-8. [PubMed]
- Marco V, Forcada P. Mesonephric adenosarcoma of the renal pelvis with heterologous elements. Am J Surg Pathol 1985;9:610-4. [Crossref] [PubMed]
- Michal M, Hes O, Bisceglia M, et al. Mixed epithelial and stromal tumors of the kidney. A report of 22 cases. Virchows Arch 2004;445:359-67. [Crossref] [PubMed]
- Picken MM, Curry JL, Lindgren V, et al. Metanephric adenosarcoma in a young adult: Morphologic, immunophenotypic, ultrastructural, and fluorescence in situ hybridization analyses: A case report and review of the literature. Am J Surg Pathol 2001;25:1451-7. [Crossref] [PubMed]
- Sameshima N, Marutsuka K, Tsukino H, et al. So-called “adenosarcoma” of the kidney a novel adult renal tumor with a cystic appearance. Pathol Int 2011;61:313-8. [Crossref] [PubMed]
- Su T, Yan F, Zhu P. Metanephric adenosarcoma: A rare case with immunohistochemistry and molecular analysis. Diagn Pathol 2014;9:179. [Crossref] [PubMed]
- Vara AR, Ruzics EP, Moussabeck O, et al. Endometrioid adenosarcoma of the bladder arising from endometriosis. J Urol 1990;143:813-5. [Crossref] [PubMed]
- N’Senda P, Wendum D, Balladur P, et al. Adenosarcoma arising in hepatic endometriosis. Eur Radiol 2000;10:1287-9. [Crossref] [PubMed]
- Jelovsek JE, Winans C, Brainard J, et al. Endometriosis of the liver containing mullerian adenosarcoma: Case report. Am J Obstet Gynecol 2004;191:1725-7. [Crossref] [PubMed]
- Brooks SE, Zhan M, Cote T, et al. Surveillance, Epidemiology, and End Results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol 2004;93:204-8. [Crossref] [PubMed]
- Arend R, Bagaria M, Lewin SN, et al. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol 2010;119:305-8. [Crossref] [PubMed]
- Stern RC, Dash R, Bentley RC, et al. Malignancy in endometriosis: Frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol 2001;20:133-9. [Crossref] [PubMed]
- Higashiura Y, Kajihara H, Shigetomi H, et al. Identification of multiple pathways involved in the malignant transformation of endometriosis Oncology Letters 2012;4:3-9. (Review). [Crossref] [PubMed]
- Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: A clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol 1990;21:363-81. [Crossref] [PubMed]
- Akhavan A, Tafti MA, Aghili F, et al. Uterine adenosarcoma in a patient with history of breast cancer and long-term tamoxifen consumption. BMJ Case Rep 2012;2012:bcr2012006590. [Crossref] [PubMed]
- Arenas M, Rovirosa A, Hernández V, et al. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer 2006;16:861-5. [Crossref] [PubMed]
- Arici DS, Aker H, Yildiz E, et al. Mullerian adenosarcoma of the uterus associated with tamoxifen therapy. Arch Gynecol Obstet 2000;264:105-7. [Crossref] [PubMed]
- Farhat F, Fakhruddine N. A case of synchronous relapse of breast cancer and uterine müllerian adenosarcoma post tamoxifen in a premenopausal woman. Eur J Gynaecol Oncol 2008;29:95-7. [PubMed]
- Jagavkar RS, Shakespeare TP, Stevens MJ. Endometrial adenosarcoma with adjuvant tamoxifen therapy for primary breast carcinoma. Australas Radiol 1998;42:157-8. [Crossref] [PubMed]
- Jessop FA, Roberts PF. Mullerian adenosarcoma of the uterus in association with tamoxifen therapy. Histopathology 2000;36:91-2. [Crossref] [PubMed]
- Le Bouëdec G, Penault-Llorca F, De Latour M, et al. Mixed müllerian tumours of the endometrium. About four cases developed on tamoxifen treatment. Gynécologie Obs Fertil 2003;31733-8.
- Martin-Loeches M, Rius J, Orti RM. Uterine sarcoma associated with tamoxifen use: Case report. Eur J Gynaecol Oncol 2003;24:202-3. [PubMed]
- Soh E, Eleti A, Jimenez-Linan M, et al. Magnetic resonance imaging findings of tamoxifen-associated uterine Müllerian adenosarcoma: a case report. Acta Radiol 2008;49:848-51. [Crossref] [PubMed]
- Chung YW, Bae HS, Han SI, et al. Endometrial mullerian adenosarcoma after toremifene treatment in breast cancer patients: A case report. J Gynecol Oncol 2010;21:269-72. [Crossref] [PubMed]
- Verschraegen CF, Arias-pulido H, Lee SJ, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: The axtell regimen. Ann Oncol 2012;23:785-90. [Crossref] [PubMed]
- Eichhorn JH, Young RH, Clement PB, et al. Mesodermal (müllerian) adenosarcoma of the ovary: A clinicopathologic analysis of 40 cases and a review of the literature. Am J Surg Pathol 2002;26:1243-58. [Crossref] [PubMed]
- Horn LC, Schmidt D, Fathke C, et al. New FIGO staging for uterine sarcomas. Pathologe 2009;30:302-3. [Crossref] [PubMed]
- Kaku T, Silverberg SG, Major FJ, et al. Adenosarcoma of the uterus: A gynecologic oncology group clinicopathologic study of 31 cases. Int J Gynecol Pathol 1992;11:75-88. [Crossref] [PubMed]
- McCluggage WG. Mullerian adenosarcoma of the female genital tract. Adv Anat Pathol 2010;17:122-9. [Crossref] [PubMed]
- Hallak M, Peipert JF, Heller PB, et al. Mullerian adenosarcoma of the uterus with sarcomatous overgrowth. J Surg Oncol 1992;51:68-70. [Crossref] [PubMed]
- Soslow RA, Ali A, Oliva E. Mullerian adenosarcomas: An immunophenotypic analysis of 35 cases. Am J Surg Pathol 2008;32:1013-21. [Crossref] [PubMed]
- Kildal W, Pradhan M, Abeler VM, et al. β-Catenin expression in uterine sarcomas and its relation to clinicopathological parameters. Eur J Cancer 2009;45:2412-7. [Crossref] [PubMed]
- Blom R, Guerrieri C. Adenosarcoma of the uterus: A clinicopathologic, DNA flow cytometric, p53 and mdm-2 analysis of 11 cases. Int J Gynecol Cancer 1999;9:37-43. [Crossref] [PubMed]
- Howitt BE, Sholl LM, Dal Cin P, et al. Targeted genomic analysis of Mullerian adenosarcoma. J Pathol 2015;235:37-49. [Crossref] [PubMed]
- Friedlander ML, Covens A, Glasspool RM, et al. Gynecologic cancer intergroup (GCIG) consensus review for mullerian adenosarcoma of the female genital tract. Int J Gynecol Cancer 2014;24:S78-82. [Crossref] [PubMed]
- Lee YJ, Kim DY, Suh DS, et al. Feasibility of uterine preservation in the management of early-stage uterine adenosarcomas: A single institute experience. World J Surg Oncol 2017;15:87. [Crossref] [PubMed]
- Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015: Featured updates to the NCCN guidelines featured updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive Cancer Network. Harborside Press 2015;13:395-404.
- Tanner EJ, Toussaint T, Leitao MM, et al. Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol Oncol 2013;129:140-4. [Crossref] [PubMed]
- Machida H, Nathenson MJ, Takiuchi T, et al. Significance of lymph node metastasis on survival of women with uterine adenosarcoma. Gynecol Oncol 2017;144:524-30. [Crossref] [PubMed]
- Jimenez WA, Sardi A, Nieroda C, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of recurrent high-grade uterine sarcoma with peritoneal dissemination. Am J Obstet Gynecol 2014;210:259.e1-8. [Crossref] [PubMed]
- Bernard B, Clarke BA, Malowany JI, et al. Uterine adenosarcomas: A dual-institution update on staging, prognosis and survival. Gynecol Oncol 2013;131:634-9. [Crossref] [PubMed]
- de Jonge MJ, Van Dam PA, Van Marck E, et al. Primary extrauterine müllerian adenosarcoma of the peritoneum. Gynecol Oncol 1995;57:126-30. [Crossref] [PubMed]
- Dincer AD, Timmins P, Pietrocola D, et al. Primary peritoneal mullerian adenosarcoma with sarcomatous overgrowth associated with endometriosis: A case report. Int J Gynecol Pathol 2002;21:65-8. [Crossref] [PubMed]
- Murugasu A, Miller J, Proietto A, et al. Extragenital mullerian adenosarcoma with sarcomatous overgrowth arising in an endometriotic cyst in the pouch of Douglas. Int J Gynecol Cancer 2003;13:371-5. [Crossref] [PubMed]
- Odunsi K, Moneke V, Tammela J, et al. Efficacy of adjuvant CYVADIC chemotherapy in early-stage uterine sarcomas: Results of long-term follow-up. Int J Gynecol Cancer 2004;14:659-64. [Crossref] [PubMed]
- Huang GS, Arend RC, Sakaris A, et al. Extragenital adenosarcoma: A case report, review of the literature, and management discussion. Gynecol Oncol 2009;115:472-5. [Crossref] [PubMed]
- Guidozzi F, Smith T, Koller AB, et al. Management of uterine Mullerian adenosarcoma with extrauterine metastatic deposits. Gynecol Oncol 2000;77:464-6. [Crossref] [PubMed]
- Liu H, Shen Z, Wu D, et al. Uterine Adenosarcoma with Sarcomatous Overgrowth: A Case Report of Aggressive Disease in a 16-Year-Old Girl and a Literature Review. J Pediatr Adolesc Gynecol 2018;31:426-31. [Crossref] [PubMed]
- Shahidsales S, Farazestanian M, Sharifi-sistani N, et al. The uterine adenosarcoma in a young woman treated by tah/bos and combined adjuvant therapy: A case report. Acta Med Iran 2020;58:138-41. [Crossref]
- Nannini M, Dondi G, Santini D, et al. A Single-Centre Experience on the Management of Adenosarcoma: A Successful Report of an Integrated Medical and Surgical Approach. Clin Med Insights Oncol 2018;12:1179554918782477. [Crossref] [PubMed]
- Wang B, Yang HD, Shi XH, et al. Advanced uterine adenosarcoma with sarcomatous overgrowth in a young woman: A case report. Medicine (Baltimore) 2019;98:e18119. [Crossref] [PubMed]
- Yuan Z, Shen K, Yang J, et al. Uterine adenosarcoma: A retrospective 12-year single-center study. Front Oncol 2019;9:237. [Crossref] [PubMed]
- Omi M, Tonooka A, Chiba T, et al. Immunohistochemical markers and the clinical course of adenosarcoma: a series of seven cases. Diagn Pathol 2020;15:119. [Crossref] [PubMed]
- Nathenson MJ, Conley AP. Prognostic factors for uterine adenosarcoma: a review. Expert Rev Anticancer Ther 2018;18:1093-100. [Crossref] [PubMed]
- Hines BJ, Porges RF, Mittal K, et al. Use of medroxyprogesterone acetate in the treatment of müllerian adenosarcoma: A case report. Gynecol Oncol 2002;85:192-5. [Crossref] [PubMed]
- Lee SJ, Bae JH, Kim DC, et al. Oral progesterone treatment in a young woman with müllerian adenosarcoma whose ovary was preserved: A case report. Int J Gynecol Cancer 2010;20:1222-4. [Crossref] [PubMed]
- Gruber TJ, Fabiano AJ, Deeb G, et al. Intracranial meningiomas in patients with uterine sarcoma treated with long-term megestrol acetate therapy. World Neurosurg 2011;76:477.e16-20. [Crossref] [PubMed]
- Tasaka N, Matsumoto K, Satoh T, et al. Therapeutic effect of dienogest on adenosarcoma arising from endometriosis: A case report. Springerplus 2013;2:618. [Crossref] [PubMed]
- Rizzo A, Pantaleo MA, Saponara M, et al. Current status of the adjuvant therapy in uterine sarcoma: A literature review. World J Clin Cases 2019;7:1753-63. [Crossref] [PubMed]
- Nathenson MJ, Conley AP, Lin H, et al. Treatment of Recurrent or Metastatic Uterine Adenosarcoma. Sarcoma 2017;2017:4680273. [Crossref] [PubMed]
- Yamagami W, Susumu N, Ninomiya T, et al. A retrospective study on combination therapy with ifosfamide, adriamycin and cisplatin for progressive or recurrent uterine sarcoma. Mol Clin Oncol 2014;2:591-5. [Crossref] [PubMed]
- del Carmen MG, Lovett D, Goodman A. A case of Müllerian adenosarcoma of the uterus treated with liposomal doxorubicin. Gynecol Oncol 2003;88:456-8. [Crossref] [PubMed]
- Maeda M, Mabuchi S, Matsumoto Y, et al. Activity of pegylated liposomal doxorubicin for extragenital mullerian adenosarcoma with sarcomatous overgrowth: A case report and a review of the literature. Eur J Gynaecol Oncol 2011;32:542-6. [PubMed]
- Roman LD, Mitchell MF, Tornos C, et al. Dedifferentiated extrauterine adenosarcoma responsive to chemotherapy. Gynecol Oncol 1993;49:389-94. [Crossref] [PubMed]
- Schroeder BA, Rodler ET, Loggers ET, et al. Clinical benefit of trabectedin in uterine adenosarcoma. Med Oncol 2013;30:501. [Crossref] [PubMed]
Cite this article as: Nigro MC, Nannini M, Rizzo A, Pantaleo MA. Current status on treatment of uterine adenosarcoma: updated literature review. Gynecol Pelvic Med 2021;4:15.




