
Sacrocolpopexy: anatomical landmarks, clinical appliance and 3-year outcomes
Introduction
Sacrocolpopexy (SCP) is the one of proven methods for the treatment of pelvic organ prolapse (POP), which is performed by cervical or vault adjustment to the anterior longitudinal ligament at the promontory. Historically, an apical correction of genital prolapse was maintained by performing a Manchester operation or sacrospinous fixation. However, several recurrent formations of enterocele after surgery pushed forward the development of a new intervention with a backward direction of vaginal tension. Sacral promontory was chosen to be the fixation point because of the dense structure of the anterior longitudinal ligament lying over it, and also its superficial, central and the highest position in the pelvis (1). Later, after wide adoption of synthetic mesh in gynecological surgery, SCP has become a “gold standard” for apical genital prolapse treatment. In its primary form, the intervention was performed using non-absorbable sutures for fixation of the cervix or vaginal cuff, but later it was evolved into the technique of synthetic mesh placement at both anterior and posterior vaginal walls (2). Implementation of laparoscopic approach into gynecological practice developed the method combining the effectiveness of promontofixation and advantages of mini-invasive surgery.
The main purpose of the intervention is to suspend cervical fibrous ring structures towards sacral promontory using synthetic mesh. There are many modifications of this seemingly standardized method, despite that, all of them are still named the same way. Using the anterior longitudinal ligament at the S1 level as the point of fixation is a crucial part of the SCP. Anatomy is the cornerstone of safe surgery, hence, it’s important to know the route of manipulations before the start to prevent any kind of complications and achieve the best result in POP treatment. In this article, we will describe step-by-step instruction of promontofixation performed either laparoscopically or robotically with the classic two-strap technique, focusing on anatomical landmarks and present our anatomical, and functional long-term outcomes covering up to 7 years of observation.
We present the following article in accordance with the STROBE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-18/rc).
Methods
Surgical technique
The timeline of the intervention could be divided into two big steps: (I) wide dissection and retroperitoneal tissue preparation combined with subtotal hysterectomy; (II) fixation of the mesh with non-absorbable sutures and peritoneal closure.
It’s always easier to work in the retroperitoneal spaces avoiding any obstacles like intestinal loops and the rectosigmoid part of the colon. Patient’s placement in Trendelenburg position often helps a lot, but in obese women, this action can be not enough to work confidently at the pelvic organs. Using laparoscopic tissue retraction systems like T-lift or placing a single monofilament fixation suture at the colon’s epiploic appendages facilitates the path to the pouch of Douglas and promontory.
Sacral promontory is a projection at the superior anterior margin of S1 vertebra and appears to be a border of the pelvic area. For better orientation at that region here are some anatomical landmarks, that lie at the same level: (I) the common iliac artery bifurcates into external and internal branches; (II) ureters change from lateral to medial side over the left external iliac artery and the right common iliac artery. Just above this level, approximately at the projection of L4 vertebra, there is bifurcation of the abdominal aorta and inferior cava vein lying behind and slightly to the right. The main point of fixation at the promontory is the anterior longitudinal ligament with thickness ranges from 1.3–2.5 mm (3). It has two layers overlying, which are: peritoneum with its areolar tissue and the presacral fascia, containing the superior hypogastric plexus and nerves. It’s always important during intervention to perform a nerve-sparing procedure to exclude negative iatrogenic effects of the surgery. Hypogastric nerve plexus, according to cadaver studies lies to the left of the midline in 75% of the patients, and only 25% goes straight at the midline (4). Hence, the dissection of the peritoneum and opening of the anterior longitudinal ligament advised approaching at the right side of the “interiliac triangle”. Borders of that anatomical landmark include edge of the sacral promontory, the right common iliac artery, and the left common iliac vein. This area contains the middle sacral artery and vein, which are branching from the posterior aspect of the abdominal aorta and inferior cava vein and lie longitudinally in the midline over the anterior longitudinal ligament (Figure 1).
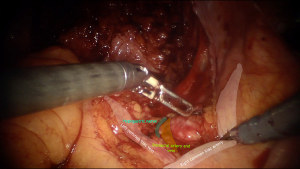
The peritoneum overlying the right common iliac artery is incised along the medial border of the artery itself for 3–4 cm starting from the promontory. Underlying presacral fascia is penetrated aside of hypogastric nerve resulting in a foramen 15 mm diameter. The peritoneal opening is extended toward the Douglas pouch forming a J-shaped incision to the left uterosacral ligament while avoiding the right hypogastric nerve and leaving it laterally. Despite the midline position or left deviation of the hypogastric nerve plexus, it has a bifurcation at the level of S1 vertebra, so the right hypogastric nerve can be met on the way from the promontory to the right uterosacral ligament. Usually, it runs in the middle between the right uterosacral ligament and the right ureter and parallel to them. It provides sympathetic innervation for contraction of the anal and urethral sphincters and carries the proprioception of the bladder, the rectum and the uterus. Therefore, a surgeon should avoid excessive use of any kind of energy during the peritoneal opening of the lateral pelvic wall and prefer sharp and blunt dissection instead.
Extra vigilance is required during detachment of the posterior vaginal wall from the rectum to prevent any kind of rectal injuries. Hence, placement of vaginal retractor can facilitate guidance during this step. Then rectovaginal space is fully opened to carry out the puborectal/pubococcygeal portion of the levator ani muscles from both sides and peritoneal body at the middle (Figure 2).
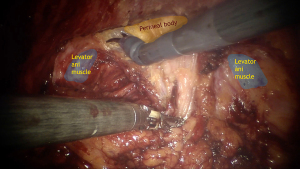
Lateral limits for this procedure are uterosacral and rectovaginal ligaments with its vessels. It’s important to avoid cut of the lateral rectal ligament, containing middle rectal vessels and the rectal nervous branch from of the inferior hypogastric plexus. Despite the point that the middle rectal artery isn’t stable presented anatomical structure, does not substantially contribute to the blood supply and considered to have only an additional impact on rectal blood flow, area of lateral rectal ligament shouldn’t be touched without need (5). There is about 80% of the posterior vaginal wall’s total length could be opened avascular and easily cleavable way (6).
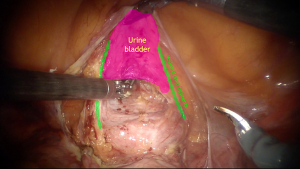
The main purpose of the vesicovaginal space opening is to create a trigone-shaped area for the anterior mesh-strap fixation to prevent or adjust upper cystocele formation caused by central or transversal defects of the Halban’s (pubocervical) fascia. Anatomical boundaries of avascular, easily cleavable space during this procedure include dorsal end of the bladder’s trigone as the lowest margin, vesicouterine ligaments as the lateral sides, and vesicouterine fold as the upper mark. It’s important to perform surgery exactly in this area because bigger descent can cause mesh-related and pharmacologically resistant voiding dysfunction. Besides, vesicouterine ligaments contain not only important vessels, such as the descending branch of the uterine artery, the superficial vesical vein (branch of the superficial uterine vein) and the cervicovesical vessels, but also branches of the bladder nerves from inferior hypogastric plexus, that are lying deeper. According to some studies, while performing it within anatomical boundaries, surgeon only can open about 1/2 of the total length of the anterior vaginal wall (6). Sometimes, it also could be difficult to dissect that area, especially in post-hysterectomy patients. Using Breisky-Navratil speculum inserted in the anterior fornix of the vagina can be quite helpful for better visualization of the vaginal wall (Figure 3). Also, assistant’s palpation of the urine bladder’s neck can mark the lower border of anterior dissection. After that, it’s preferable to perform subtotal hysterectomy with the uterine appendages or the fallopian tubes instead of placing the mesh at the uterus, because of lifetime risk for cysts, hyperplastic process or malignancies that could become the indication for subsequent interventions, so the synthetic straps will lead to technical difficulties during secondary surgery. Nowadays there are controversial points of view about concomitant total hysterectomy, but some research results in equal short-time outcomes as in subtotal hysterectomy cases (7,8).
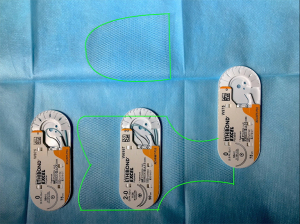
At the end of this part and only at that time of the intervention surgeon should extract the mesh from the sterile package and cut it forming two straps, posterior and anterior, approximately 10×15 and 7.5×5 cm long respectively in a special way (Figure 4). Anterior patch has three plain sides and an arrow-shaped one, that should look similar to the anterior dissection bottom line. The posterior strap in its turn represents two different parts: narrow rectangular loose end and wide “pelt-like” body of the mesh. Loose end (about 1.5–2.0 cm width) is maintained to be thereafter sutured at the promontory. To define its right length, the surgeon always should be provided with information on patient’s pre-op total vaginal length (TVL) measurement found during a bimanual examination without anesthesia effect, so the assistant during intervention could elevate the cervix or vaginal cuff at the safe and physiological level. The distance between the sacral promontory and the top point of adjusted prolapse will be the right length of the sacral part of the mesh. The width of the posterior strap also could be anatomically based on the distance between levator ani muscles palpated vaginally and its lower border should always be curved to prevent any rectal compression by the mesh in the future.
Posterior strap then inserted in the peritoneal cavity and fixed upwards. Firstly, it sutured to the middle portion of puborectal muscles with a single non-absorbable interrupted suture from both sides (Ethibond Excel #0, Ethicon, J&J, Belgium). Then it fastened to the perineal body, uterosacral ligaments, posterior vaginal wall, and vaginal cuff/uterine cervix also using non-absorbable sutures (Ethibond Excel #2/0, Ethicon, J&J, Belgium). Recent study exploring the finite element (FE) model of the female pelvic system during abdominal sacral colpopexy concluded that recommended spacing between sutures of the posterior compartment should be about 3.5 cm (9). Anterior strap then inserted in the peritoneal cavity and gently fixed to the anterior vaginal wall upwards from the bladder’s trigone projection zone by non-absorbable polyester interrupted sutures (Ethibond Excel #2/0, Ethicon, J&J, Belgium). Recommended spacing between sutures in that compartment is 2.5 cm (9). Then, posterior and anterior straps should be sutured together without breaking longitudinal position of the narrow line of the posterior one (Figure 5).
Loose end of the back strap then fixed using non-absorbable suture to the anterior longitudinal ligament in avascular area (Ethibond Excel #0, Ethicon, J&J, Belgium) (Figure 6). As we mentioned above, surgeon should always keep in mind, that average thickness of the anterior longitudinal sacral ligament is no more than 2 mm. Higher depth of fixation can cause higher risk of osteomyelitis and spondylodiscitis formation during post-op period. That statement should also prevent using staples at that area because of deeper penetration of the ligament (5 mm) compared to 2–3 mm using non-absorbable sutures (10). Also, according to some studies, staples have lower biomechanical resistance than non-absorbable sutures and it’s always needed more than one staple to avoid mesh snapping off (11).
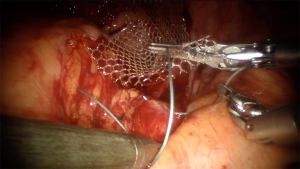
Mesh peritonization always should be performed as a prevention of adhesion formation. For that instance, we use continuous absorbable monofilament or barbed sutures (Monocryl #0, Ethicon, J&J, Belgium; V-loc 180 #2/0, Covidien, Medtronic, MN, USA) (Figure 7). Urethral catheter and vaginal gauze then placed for 24 hours after surgery.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Moscow Regional Scientific Research Institute of Obstetrics and Gynecology (No.: 06004245) and informed consent was taken from all individual participants.
Statistical analysis
This was a descriptive longitudinal study and standard statistical analyses were performed. Categorical data were presented as % (n/N) and continuous data were presented as mean ± standard deviation or median (range).
For the comparison of continuous variables was used Wilcoxon signed rank test and paired t-test with confidence interval (CI): 95%. Statistical significance was defined at P<0.05. Calculations were performed in IBM® SPSS® Statistics 23 (IBM Corporation, Armonk, NY, USA).
Sample size was defined to be that of all eligible patients during the period of retrospective study cohort. No study of potential bias was set in the scope of present manuscript.
Outcomes
During 2013–2020 years in Moscow Regional Scientific Research Institute of Obstetrics and Gynecology SCP was performed in 387 patients, 193 using laparoscopic approach and 194 robotic-assisted. Demographic data was collected including age, body mass index (BMI), parity, menopausal status, sexual activity and prior surgery. All women were white Caucasian females, which were parous in 99.3%. Mean age was 58.6 years (range, 32–83 years), 84 (21.7%) patients were normal weighted, 217 (56.1%) were overweighted, 86 (22.2%) were obese. Preoperative evaluation included detailed urogynecologic history and physical examination. Perioperative collected data is presented in Table 1.
Table 1
| Characteristics | Value |
|---|---|
| Age, y | 58.6±7.9 |
| BMI, kg/m2 | 27.7±2.8 |
| Multiparity, % | 61.9 |
| Postmenopausal, % | 84.5 |
| Sexually active, % | 55.8 |
| Prior hysterectomy, % | 29.7 |
| Prior prolapse surgery, % | 26.5 |
| Urinary incontinence, % | 33.0 |
| Urgent incontinence, % | 29.5 |
| Mixed incontinence, % | 16.5 |
| Operative time, min | 142.9±29.9 |
| Blood loss, mL | 86.8±35 |
| Concomitant incontinence surgery, % | 3.1 |
| Concomitant Burch colposuspension, % | 2.6 |
| Concomitant sub-urethral sling, % | 0.5 |
BMI, body mass index.
All bimanual examination results before and after the intervention were evaluated using the POP-quantification (POP-Q) system (12). SCP was performed in patients with apical or combined severe forms of prolapse grades III–IV according to POP-Q. All surgery was performed by gynecologists, that already had laparoscopic surgery experience more than 10 years and made more than 40 SCP interventions at the time of 2013, so we’ve excluded learning curve interference from our results.
All patients were asked to continue follow-up after surgery with the first check up at 3 months after intervention and then were examined each year. Follow-up included physical examination and the last measurements then were used for statistical analysis. We’ve included women who underwent SCP in 2013–2020 years period. The mean range of observation was 41.4±31.3 months (range, 3–92 months). For recurrence of POP, we took the criteria that were previously stated by International UroGynecological Association: direct or indirect POP reaching or going below the level of the hymen (POP-Q ≥ stage 2b) for objective recurrence and having symptoms attributed to recurrent POP for subjective recurrence (13).
Functional outcomes were evaluated by international validated questionnaires. The summary comparison performed before and after surgery during each control examination. Also, in that case we used the last results for further analysis. Each questionnaire has a minimal clinical important difference (MCID) in points, after overcoming which it is considered that reliable success of the functional result of surgical correction has been achieved. Three different questionaries were used: Pelvic Floor Distress Inventory-20 (PFDI-20), MCID-24 (14) assessing the severity of pelvic floor dysfunction; Pelvic Floor Inventory Questionnaire-7 (PFIQ-7), MCID-37 (15) assessing POP influence on social and private life; Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12 (PISQ-12), MCID-6 (16) assessing sexual life disorders caused by pelvic floor dysfunction.
Results
Three hundred and fifty-seven of patients were assessed post-op during an examination at the ward 1 year and more after the SCP. POP recurrence appeared in 29 cases (8.1%). Most of them resulted in Ba prolapse cases (n=26, 7.3%). There were only 4 (1.1%) Bp prolapse recurrence patients and 2 (0.6%) patients with C prolapse recurrence. Clinical improvement on sex life based on PISQ-12 score was met in 132 (66.3%) patients. Two hundred and fifty (70.0%) of women met the criteria of MCID during the assessment of pelvic floor dysfunction (PFDI-20). Two hundred and five (57.4%) of patients improved their social life according to PFIQ-7 score. Nine cases (2.3%) of stress urinary incontinence de novo formation were observed. Thirty-eight (21.3%) women had full resolve of their incontinence after surgery without providing any concomitant anti-stress procedures. Only 34 (19.1%) of women, having stress urinary incontinence before SCP came for additional anti-stress surgical correction. Table 2 shows a comparison of anatomical and functional outcomes before surgery with the last variables after surgery.
Table 2
| Characteristics | Prior surgery (mean ± SD) | After surgery (mean ± SD) | P value |
|---|---|---|---|
| Ba point, cm | 2.1±1.9 | −1.5±1.0 | <0.05 |
| C point, cm | 4.1±2.2 | −6.8±1.1 | <0.05 |
| Bp point, cm | 3.0±1.5 | −2.4±0.6 | <0.05 |
| PFDI-20 | 115.0±43.8 | 65.3±38.6 | <0.05 |
| PFIQ-7 | 71.6±26.8 | 23.4±17.3 | <0.05 |
| PISQ-12 | 20.1±5.8 | 29.0±8.4 | <0.05 |
PFDI-20, Pelvic Floor Distress Inventory-20; PFIQ-7, Pelvic Floor Inventory Questionnaire-7; PISQ-12, Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire-12.
Discussion
Our long-term observation found good physical and functional results for SCP. Despite anatomical restrictions in covering the anterior vaginal wall, statistically significant improvement was stated in all three compartments. The majority of patients have met MCID threshold for functional success in validated questionaries measuring symptoms, social and sexual life. Covering a population of 387 women can definitely let us assume, that SCP is truly complex and effective in multi-compartment POP. Further evaluation is needed for durability assessment of the intervention. Our main limitations were the single-center base of our study and also, in our opinion, it would be more descriptive to provide comparison studies between different methods or approaches (ex. vaginal and abdominal route) and even between variations of SCP techniques. Also, a more thorough evaluation of urinary disorders could possibly make a conclusion about the suitability of concomitant anti-stress surgery performed.
Conclusions
Sacral colpopexy is a historically justified and approved technique of the apical POP treatment. Knowledge of the anatomical landmarks and surgical pathway during surgery can impressively decrease intra- and post-op iatrogenic complications. Our long-term results reflect, that this method may not be advised at POP cases with anterior-apical prolapse, because of the higher prevalence of cystocele formation (7.3%) comparing to apical and posterior recurrence, and also surgical imperfection of safe and avascular dissection of the anterior vaginal wall. However, in the patients with vaginal vault prolapse, complex and posterior-apical prolapse, such intervention still should be considered as a “gold standard”.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gynecology and Pelvic Medicine for the series “Surgical Treatment of Genital Prolapse and Urinary Incontinence”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-18/rc
Data Sharing Statement: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-18/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-18/coif). The series “Surgical Treatment of Genital Prolapse and Urinary Incontinence” was commissioned by the editorial office without any funding or sponsorship. AP served as an unpaid Guest Editor of the series and serves as an unpaid editorial board member of Gynecology and Pelvic Medicine from August 2020 to July 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Moscow Regional Scientific Research Institute of Obstetrics and Gynecology (No.: 06004245) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ARTHURE HG. SAVAGE D. Uterine prolapse and prolapse of the vaginal vault treated by sacral hysteropexy. J Obstet Gynaecol Br Emp 1957;64:355-60. [Crossref] [PubMed]
- LANE FE. Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol 1962;20:72-7. [Crossref] [PubMed]
- White AB, Carrick KS, Corton MM, et al. Optimal location and orientation of suture placement in abdominal sacrocolpopexy. Obstet Gynecol 2009;113:1098-103. [Crossref] [PubMed]
- Shiozawa T, Huebner M, Hirt B, et al. Nerve-preserving sacrocolpopexy: anatomical study and surgical approach. Eur J Obstet Gynecol Reprod Biol 2010;152:103-7. [Crossref] [PubMed]
- Kiyomatsu T, Ishihara S, Murono K, et al. Anatomy of the middle rectal artery: a review of the historical literature. Surg Today 2017;47:14-9. [Crossref] [PubMed]
- Ercoli A, Campagna G, Delmas V, et al. Anatomical insights into sacrocolpopexy for multicompartment pelvic organ prolapse. Neurourol Urodyn 2016;35:813-8. [Crossref] [PubMed]
- Pan K, Cao L, Ryan NA, et al. Laparoscopic sacral hysteropexy versus laparoscopic sacrocolpopexy with hysterectomy for pelvic organ prolapse. Int Urogynecol J 2016;27:93-101. [Crossref] [PubMed]
- Maldonado PA, Norris KP, Florian-Rodriguez ME, et al. Sacrocolpopexy With Concomitant Total vs Supracervical Hysterectomy: Functional Support Comparisons in Cadavers. Female Pelvic Med Reconstr Surg 2019;25:213-7. [Crossref] [PubMed]
- Jeanditgautier E, Mayeur O, Brieu M, et al. Mobility and stress analysis of different surgical simulations during a sacral colpopexy, using a finite element model of the pelvic system. Int Urogynecol J 2016;27:951-7. [Crossref] [PubMed]
- Boukerrou M, Orazi G, Nayama M, et al. Promontofixation procedure: use of non-absorbable sutures or Tackers? J Gynecol Obstet Biol Reprod (Paris) 2003;32:524-8. [PubMed]
- Gadonneix P, Ercoli A, Scambia G, et al. The use of laparoscopic sacrocolpopexy in the management of pelvic organ prolapse. Curr Opin Obstet Gynecol 2005;17:376-80. [Crossref] [PubMed]
- Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10-7. [Crossref] [PubMed]
- Ismail S, Duckett J, Rizk D, et al. Recurrent pelvic organ prolapse: International Urogynecological Association Research and Development Committee opinion. Int Urogynecol J 2016;27:1619-32. [Crossref] [PubMed]
- Karjalainen PK, Mattsson NK, Jalkanen JT, et al. Minimal important difference and patient acceptable symptom state for PFDI-20 and POPDI-6 in POP surgery. Int Urogynecol J 2020; Epub ahead of print. [Crossref] [PubMed]
- Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol 2005;193:103-13. [Crossref] [PubMed]
- Mamik MM, Rogers RG, Qualls CR, et al. The minimum important difference for the Pelvic Organ Prolapse-Urinary Incontinence Sexual Function Questionnaire. Int Urogynecol J 2014;25:1321-6. [Crossref] [PubMed]
Cite this article as: Popov A, Klyushnikov I, Fedorov A, Koval A, Tyurina S, Idashkin A. Sacrocolpopexy: anatomical landmarks, clinical appliance and 3-year outcomes. Gynecol Pelvic Med 2022;5:5.




