
Uterine adenomyoma—what we know, and what we don’t know: a narrative review
Introduction
Adenomyosis is a benign disorder of the uterus characterised by infiltration of endometrial glands and stroma into the myometrium, with reactive hypertrophy of the surrounding smooth muscle cells of the myometrium, affecting up to 20.9% of women at reproductive age attending gynaecology clinics (1). It is a heterogeneous disease that can present in different configurations in the myometrium and is considered diffuse when numerous foci of endometrial glands and stroma are dispersed in the myometrium, or focal when circumscribed nodular aggregates are observed (2). Adenomyosis of the outer myometrium corresponds to lesions separated from the junctional zone, the subendometrial myometrium. The junctional zone is a concept which originates from magnetic resonance imaging (MRI) imaging modality as it appears hypoechoic compared to the endometrium and myometrium. The exact corresponding tissue in anatomical terms is not clearly known, but the thickness of the junctional zone appears to correspond to the diagnosis of adenomyosis, hence it is a frequently used term in adenomyosis-related texts. Adenomyosis of the inner myometrium is thought to be characterized by endometrial implants scattered throughout the myometrium and enlargement of the junctional zone (3).
Uterine adenomyomas are areas of focal adenomyosis with additional compensatory hypertrophy of the surrounding myometrium (4). They are comprised of a circumscribed nodular aggregate of benign endometrial glands surrounded by endometrial stroma with leiomyomatous smooth muscle bordering the endometrial stromal component (5). Adenomyomas may be located within the myometrium or originate in the endometrium and grow as polyps. Uterine adenomyomas were first described in 1896 and a more detailed description appeared in 1918, but they have since received relatively little attention and the available literature on this topic is sparse (6-8). The purpose of this narrative review is to address what is known about uterine adenomyomas, and what is not yet known. We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-50/rc).
Methods
We searched MEDLINE (through PubMed) and EMBASE (through Embase.com) for potentially eligible records using a combination of MeSH (Medical subject Headings) and relevant index terms, Table 1. We used MeSH or index terms for the following key words: “adenomyosis” and “adenomyoma”. The search was performed on 1st August 2021 and limited to humans and papers in the English language. Relevant articles were chosen by the authors and additional relevant references were included from the reference list of these articles.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 1st August 2021 |
| Databases and other sources searched | Medline, Embase |
| Search terms used (including MeSH and free text search terms and filters) | MeSH terms and index words used for “adenomyosis” and “adenomyoma” |
| Timeframe | 1/8/2021–8/8/2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Narrative review. Search limited to human papers in English language |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Selection of relevant articles conducted independently by SL and ES |
Epidemiology
A retrospective cohort study from the United States with data from a mixed-model health insurance and care delivery system in 333,693 women aged 16–60 years found that the overall incidence of adenomyosis was 1% (9). The incidence was highest for women aged 40–45 years, with women in their early 40s most likely to have symptomatic adenomyosis. Incidence rates were found to be disproportionately high among black women. The coexistence of endometriosis and fibroids was common, at 18% and 47%, respectively. The healthcare burden associated with adenomyosis was substantial, with 82% of women requiring hysterectomy and 38% using chronic pain medication (9). Whilst adenomyosis was previously diagnosed almost exclusively at the time of hysterectomy, modern ultrasound techniques now allow for non-invasive diagnosis.
Pathogenesis
Several theories exist with regards to how adenomyosis develops, though its pathogenesis is not yet fully understood. The most widespread hypothesis is the invagination theory, in which basalis endometrium infiltrates into the myometrium through an abnormal junctional zone. Tissue injury and repair (TIAR) is proposed as the primary mechanism for myometrial infiltration, in which chronic myometrial contractions induce microtrauma at the endometrial-myometrial interface, resulting in inflammation and a subsequent increase in oestrogen production, followed by repeated cycles of trauma occurring through a positive feedback mechanism (10,11). A second theory suggests there is metaplasia of ectopic intramyometrial mullerian rests that occurs de novo (12).
It has been suggested that three different pathophysiological processes take place based on where the adenomyosis is located. Distinct expression patterns of fibrosis-related proteins exist in inner and outer adenomyosis, suggesting invasion of the eutopic endometrium through the myometrium for inner adenomyosis and invasion of ectopic endometrial cells in the myometrium for outer focal lesions from adjacent endometriosis lesions (13).
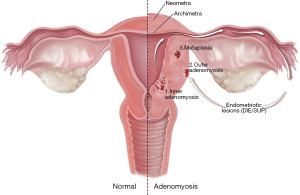
A third type in which adenomyosis is located intramurally without involvement of the junctional zone or serosa has also been described, postulated to represent metaplasia (13). A correlation has also been noted between focal lesions of the outer myometrium and the presence of deep infiltrating endometriosis (14). Chapron et al. propose an “outside to inside invasion” theory, hypothesizing the migration of ectopic endometrial cells from posterior endometriosis nodules into the myometrium, see Figure 1. One research group studied the tissue distribution patterns in women with inner adenomyosis and women with outer adenomyosis or coexisting deep infiltrating endometriosis. They noted a similar pattern of tissue in women with outer adenomyosis and coexisting deep infiltrating endometriosis lesions, whereas women with inner adenomyosis were noted to have glands and stromal cells in similar distribution patterns to the endometrium (15). A recent observational study concluded that inner and outer adenomyosis ought to be considered as two different entities as they found that these two forms were associated with distinct clinical presentations, as described below (3).
Clinical presentation
The clinical presentation in women with adenomyosis is heterogeneous, with women experiencing symptoms of dysmenorrhoea, pelvic pain, heavy menstrual bleeding (HMB), infertility, and a third remaining asymptomatic (5,16). The difference in clinical presentation of women with inner and outer adenomyosis has been studied (3,13). Inner adenomyosis was found to be more common in older, multiparous women with a raised body mass index (BMI) and previous history of uterine surgery, whereas outer adenomyosis was more common in younger women and was independently associated with the presence of deep infiltrating endometriosis. Both phenotypes gave rise to pelvic pain and dysmenorrhea, with no difference in intensity. Women with diffuse adenomyosis more commonly experienced HMB, even after exclusion of those with co-existing leiomyomas, whereas women with focal adenomyosis, often referred to as intramural adenomyomas by pathologists, were more likely to experience primary infertility (17).
A cross-sectional study in 496 women undergoing surgery for benign gynaecological conditions examined the impact of focal and diffuse adenomyosis on assisted conception outcomes (17). One third of women with adenomyosis in this cohort experienced infertility. They noted that 96.3% of women with focal adenomyosis had co-existing endometriosis, compared to 61.5% in women with diffuse adenomyosis. Focal adenomyosis was found to be an independent associated factor for primary infertility. This association may not be causal in relationship and there remains a need for further studies to evaluate the impact of adenomyosis on fertility and assisted conception.
Several meta-analyses have suggested that women with adenomyosis have a lower clinical pregnancy rate with a higher miscarriage rate (16,18-20). A multi-centre observational study found that the probability of clinical pregnancy decreased from 42.7% in women with no adenomyosis to 22.9% in those with four features of adenomyosis on ultrasound. Women with all seven ultrasound features of adenomyosis had an estimated clinical pregnancy rate of 13.0%, suggesting that reproductive outcome correlates closely with severity of adenomyosis (21).
There are several proposed theories to explain the negative impact of adenomyosis on fertility, including disturbed uterotubal transport of sperm and abnormal uterine peristalsis (22).
Myometrial abnormalities in women with adenomyosis cause raised intrauterine pressure and loss of normal rhythmic contractions, resulting in hyperperistalsis (23). Molecular changes in the endometrium of women with adenomyosis include increased levels of inflammatory markers and oxidative stress, and reduced expression of implantation markers, leading to impaired implantation (22).
Diagnosis—transvaginal ultrasonography (TVS)/MRI
Recent advances in ultrasound and MRI technology have facilitated the non-invasive diagnosis of adenomyosis, avoiding the need for surgical excision to make a diagnosis. A recent systematic review and meta-analysis evaluating imaging techniques for diagnosing adenomyosis reported the sensitivity for MRI, two-dimensional transvaginal ultrasonography (2D-TVS), and three-dimensional transvaginal ultrasonography (3D-TVS) to be 78%, 74%, and 84% respectively and specificity of 88%, 76%, and 84%, respectively (24). It was concluded that all modalities offer sufficient quality for adenomyosis diagnosis. In comparison to 2D-TVS, 3D-TVS improved the quality of adenomyosis diagnosis by enabling improved detection of changes at the endometrial-myometrial junction, which was a key diagnostic determinant (24). Multiple classifications for adenomyosis have been proposed, but there is currently no consensus on which to adopt for clinical practice and there remains a need for uniform terminology and consensus classification (2,13,25-29).
Adenomyomas are seen as a myometrial mass with indistinct margins on MRI and low signal intensity on all MRI sequences. The most common feature of adenomyosis on MRI is thickening of the junctional zone, with a thickness exceeding 12 mm being highly predictive of the diagnosis (30). On TVS, adenomyomas are seen as a focal region of adenomyosis appearing as a myometrial mass, which may be difficult to distinguish from a uterine fibroid. The degree to which the contour of the uterus is distorted is less marked in an adenomyoma, margins are indistinct and blend with the surrounding myometrium and there is translesional flow of vascularity, whereas fibroids are typically well-defined with a pseudocapsule of surrounding myometrial tissue and circumferential flow of vascularity (2,31). Associated features of adenomyosis on TVS include asymmetrical myometrial thickening, parallel shadowing, myometrial cysts, an irregular endometrial-myometrial junction, linear striations and hyperechoic islands (1). A study evaluating ultrasound prevalence of adenomyosis and uterine fibroids in women with endometriosis found 3.1% of women with endometriosis to have an ultrasound diagnosis of fibroids, 21.2% to have an ultrasound diagnosis of adenomyosis and 14.6% had both uterine disorders co-existing with endometriosis (32).
Treatment
Adenomyosis can negatively impact a woman’s quality of life due to symptoms of dysmenorrhoea, pelvic pain, HMB and infertility. Considerations for treatment include a woman’s age, reproductive status and clinical symptoms. Evidence for the effectiveness of medical and surgical treatment options in women with adenomyosis remains limited.
Adenomyosis is an oestrogen-dependent disease that may respond to medical treatment with a transient or sustained improvement in symptoms. Medical treatment options include non-hormonal agents such as non-steroidal anti-inflammatory drugs and hormonal agents such as progestins, combined oral contraceptives, danazol and gonadotrophin releasing hormone (GnRH) analogues. Progestins have an anti-proliferative and anti-inflammatory effect, causing decidualisation and subsequent endometrial atrophy. A randomised controlled trial comparing dienogest with placebo in women with adenomyosis found it was effective in controlling pain and heavy bleeding, with treatment remaining effective after one year (33).
The levonorgestrel intrauterine system (LNG-IUS) is an effective, long-acting reversible treatment which has been shown to reduce menstrual bleeding, pain, and uterine volume (34). It has a direct local action on adenomyotic foci and causes endometrial atrophy (35). Combined oral contraceptives may offer effective symptom control in women with adenomyosis, though there are no randomized controlled trials to support its use in this population. There is limited evidence on the systemic treatment of adenomyosis with danazol, due to the high incidence of androgenic side effects. Danazol administered as vaginal tablets and intra-cervical injections has been shown to be effective in reducing HMB and pain in women with adenomyosis (36,37).
When medical treatment is unsuccessful and surgery is not desired, GnRH analogues with hormone replacement therapy (HRT) can be used as a long-term treatment option until the age of menopause. Hypogonadic side effects include a negative impact on bone and cardiovascular health, vasomotor syndrome, genital atrophy and mood instability. These can be managed with HRT as add-back therapy. Continuous prolonged treatment with GnRH analogues results in an initial stimulatory effect, followed by central downregulation and inhibition of the hypothalamo-pituitary-ovarian axis, suppressing ovarian function and inducing a hypoestrogenic state. GnRH analogues have a direct effect on adenomyosis, causing suppression of tissue inflammatory reaction, angiogenesis and cell proliferation and inducing apoptosis (15). They have been shown to cause a significant reduction in uterine volume and an improvement in HMB and pelvic pain (38-40). Ongoing research aims to evaluate the role of selective estrogen receptor modulators, selective progesterone receptor modulators, aromatase inhibitors, anti-platelet agents, valproic acid and GnRH antagonist in treating women with adenomyosis (35). Minimally invasive procedures can be offered when medical therapy is ineffective, though there remains limited evidence for the optimal treatment in relation to adenomyosis lesion characteristics, symptom severity and reproductive aspirations of a woman.
Uterine artery embolization (UAE) is an established treatment option for women with uterine fibroids (41). Two meta-analyses have shown that UAE is also an effective long-term treatment for women with adenomyosis experiencing HMB and dysmenorrhea, though it is not suitable for women who wish to preserve fertility (42,43). Treatment aims to induce post-procedure necrosis of ectopic endometrial tissue. One study suggested that women with focal or diffuse adenomyosis have an equivalent response (44).
High intensity focused ultrasound (HIFU) generates a high intensity acoustic beam that is precisely focused on a target area with MRI guidance, inducing focal thermocoagulation of adenomyotic lesions. It is used successfully in the treatment of uterine fibroids. Pooled results from trials examining the effectiveness of HIFU in women with adenomyosis showed that 88% of 669 women experienced symptom relief at 12 months (41). Distance to beam, size of lesion and reproductive aspirations are important considerations for patient selection. Further studies are needed before HIFU can be used as an established treatment option for adenomyosis, particularly in women wishing to conceive in the future.
Endometrial ablation is another treatment option in women with adenomyosis and HMB or dysmenorrhoea when medical treatment is insufficient for relief of symptoms. One study showed that the presence of adenomyosis or fibroids quadrupled the risk of failure after endometrial ablation (45), whereas other studies have not shown this association (46,47). Further data on outcomes of endometrial ablation in women with adenomyosis are needed.
Hysterectomy is the definitive surgical treatment for adenomyosis. In women with focal adenomyosis wishing to preserve their reproductive potential, uterine-sparing surgery such as adenomyomectomy is an option. Uterine-sparing surgery in women with adenomyosis aims to remove adenomyotic tissue, preserve a functional uterus and minimise the risk of uterine rupture in a subsequent pregnancy. Complete or partial excision may be performed laparoscopically by a classic myomectomy technique. The uterine wall is repaired in two or more layers and can be reconstructed using various techniques. The incisional cavity requires closure without leaving dead space and creation of a uterine wall with sufficient thickness, though there is no data to confirm that the uterine wall thickness determines the strength of the scar (48). Repair of the uterine defect following uterine-sparing surgery for adenomyosis can be more difficult than for uterine fibroids, with reduced tensile strength of the myometrium due to adenomyotic foci. The decreased tensile strength of the uterus increases risk of uterine rupture in a future pregnancy. Previously described techniques include transverse H incision, U-shaped suturing of the myometrium, overlapping flaps and double or triple flaps (49-54). Combing surgery with GnRH analogue treatment may improve its effectiveness (55).
There remains no consensus on the optimal management of infertile women with adenomyomas undergoing ART. Several retrospective studies evaluating the benefit of prolonged downregulation with GnRH analogue prior to frozen thawed embryo transfer have reported improved reproductive outcomes, whereas one retrospective study found a negative impact and another showed no difference in outcomes (56-60). One study in infertile women with adenomyosis undergoing fresh embryo transfer evaluated prolonged downregulation with GnRH analogue for 2–4 months, reporting a significantly higher clinical pregnancy rate and live birth rate in women with diffuse adenomyosis who used the prolonged GnRHa protocol. However, in women with focal adenomyosis no difference in reproductive outcomes was found between the two arms (60). Ongoing prospective randomised controlled trials evaluating the effectiveness of prolonged downregulation with GnRH analogues in women with adenomyosis undergoing IVF will shed further light regarding its true impact (61-63).
Summary
Adenomyosis is present in a significant proportion of women presenting with HMB and infertility. Current research is limited regarding its effect on fertility, but available data suggests it has a detrimental impact on natural fertility and fertility treatment outcomes. Its involvement in HMB is better established. Historically treatment was limited to hysterectomy but the contemporary approach includes a number of medical and surgical options to preserve fertility and the uterus.
Acknowledgments
We thank Alex Webber of DNA Illustrations for producing Figure 1. The pathogenesis of adenomyosis (www.dnaillustrations.com).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Omer Lutfi Tapisiz and Sadiman Kiykac Altinbas) for the series “Uterine Fibroids: Various Aspects with Current Perspectives” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-50/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-50/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-50/coif). The series “Uterine Fibroids: Various Aspects with Current Perspectives” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 2012;27:3432-9. [Crossref] [PubMed]
- Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284-98. [Crossref] [PubMed]
- Bourdon M, Oliveira J, Marcellin L, et al. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod 2021;36:349-57. [Crossref] [PubMed]
- Fox H, Wells M. Haines, Taylor. Obstetrical and Gynaecological Pathology. (4th edn), (eds). Churchill Livingstone, 1995.
- Peric H, Fraser IS. The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006;20:547-55. [Crossref] [PubMed]
- Von Recklinghausen F. Die adenomyome und cytadenome der uterus undtubenwandung, ihre abkunft von resten des wolff`schen korpers. Berlin; 1896.
- Cullen TS. Adenomyoma uteri diffusum beningum. Johns Hopkins Hosp Rep 1896;6:133-54.
- Lockeyer C. Fibroids and Allied Tumours (Myoma and Adenomyoma); Their Pathology, Clinical Features and Surgical treatment. London: Macmillan and Co, 1918.
- Yu O, Schulze-Rath R, Grafton J, et al. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am J Obstet Gynecol 2020;223:94.e1-94.e10. [Crossref] [PubMed]
- Thompson JR, Davion RJ. Adenomyosis of the uterus: an enigma. J Natl Med Assoc 1986;78:305-7. [PubMed]
- Brosens I, Derwig I, Brosens J, et al. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod 2010;25:569-74. [Crossref] [PubMed]
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update 1998;4:312-22. [Crossref] [PubMed]
- Kishi Y, Suginami H, Kuramori R, et al. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol 2012;207:114.e1-7. [Crossref] [PubMed]
- Chapron C, Tosti C, Marcellin L, et al. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum Reprod 2017;32:1393-401. [Crossref] [PubMed]
- Khan KN, Kitajima M, Hiraki K, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod 2010;25:642-53. [Crossref] [PubMed]
- Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 2014;29:964-77. [Crossref] [PubMed]
- Bourdon M, Santulli P, Oliveira J, et al. Focal adenomyosis is associated with primary infertility. Fertil Steril 2020;114:1271-7. [Crossref] [PubMed]
- Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril 2017;108:483-490.e3. [Crossref] [PubMed]
- Horton J, Sterrenburg M, Lane S, et al. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update 2019;25:592-632. [Crossref] [PubMed]
- Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: a systematic review and meta-analysis. Reprod Biomed Online 2021;42:185-206. [Crossref] [PubMed]
- Mavrelos D, Holland TK, O'Donovan O, et al. The impact of adenomyosis on the outcome of IVF-embryo transfer. Reprod Biomed Online 2017;35:549-54. [Crossref] [PubMed]
- Mehasseb MK, Bell SC, Pringle JH, et al. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil Steril 2010;93:2130-6. [Crossref] [PubMed]
- Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017;35:592-601. [Crossref] [PubMed]
- Tellum T, Nygaard S, Lieng M. Noninvasive Diagnosis of Adenomyosis: A Structured Review and Meta-analysis of Diagnostic Accuracy in Imaging. J Minim Invasive Gynecol 2020;27:408-418.e3. [Crossref] [PubMed]
- Gordts S, Brosens JJ, Fusi L, et al. Uterine adenomyosis: a need for uniform terminology and consensus classification. Reprod Biomed Online 2008;17:244-8. [Crossref] [PubMed]
- Lazzeri L, Morosetti G, Centini G, et al. A sonographic classification of adenomyosis: interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil Steril 2018;110:1154-1161.e3. [Crossref] [PubMed]
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril 2014;101:472-87. [Crossref] [PubMed]
- Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril 2018;109:389-97. [Crossref] [PubMed]
- Exacoustos C, Morosetti G, Conway F, et al. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J Minim Invasive Gynecol 2020;27:1308-15. [Crossref] [PubMed]
- Larsen SB, Lundorf E, Forman A, et al. Adenomyosis and junctional zone changes in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol 2011;157:206-11. [Crossref] [PubMed]
- Brindha D, Kandaswamy A. LakshmiDeepika C. Digital image analysis of uterine ultrasound for classification of uterine myoma and adenomyoma. J Med Imaging Health Inform 2015;5:1603-6. [Crossref]
- Capezzuoli T, Vannuccini S, Fantappiè G, et al. Ultrasound findings in infertile women with endometriosis: evidence of concomitant uterine disorders. Gynecol Endocrinol 2020;36:808-12. [Crossref] [PubMed]
- Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril 2017;108:673-8. [Crossref] [PubMed]
- Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189-93. [Crossref] [PubMed]
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril 2018;109:398-405. [Crossref] [PubMed]
- Takebayashi T, Fujino Y, Umesaki N, et al. Danazol suspension injected into the uterine cervix of patients with adenomyosis and myoma. Preliminary study. Gynecol Obstet Invest 1995;39:207-11. [Crossref] [PubMed]
- Luisi S, Razzi S, Lazzeri L, et al. Efficacy of vaginal danazol treatment in women with menorrhagia during fertile age. Fertil Steril 2009;92:1351-4. [Crossref] [PubMed]
- Grow DR, Filer RB. Treatment of adenomyosis with long-term GnRH analogues: a case report. Obstet Gynecol 1991;78:538-9. [PubMed]
- Takeuchi A, Koga K, Miyashita M, et al. Dienogest reduces proliferation, NGF expression and nerve fiber density in human adenomyosis. Eur J Obstet Gynecol Reprod Biol 2016;207:157-61.22.
- Nelson JR, Corson SL. Long-term management of adenomyosis with a gonadotropin-releasing hormone agonist: a case report. Fertil Steril 1993;59:441-3. [Crossref] [PubMed]
- Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol 2018;51:119-37. [Crossref] [PubMed]
- de Bruijn AM, Smink M, Lohle PNM, et al. Uterine Artery Embolization for the Treatment of Adenomyosis: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol 2017;28:1629-1642.e1. [Crossref] [PubMed]
- Popovic M, Puchner S, Berzaczy D, et al. Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol 2011;22:901-9; quiz 909. [Crossref] [PubMed]
- Bae SH, Kim MD, Kim GM, et al. Uterine Artery Embolization for Adenomyosis: Percentage of Necrosis Predicts Midterm Clinical Recurrence. J Vasc Interv Radiol 2015;26:1290-6.e2. [Crossref] [PubMed]
- Wishall KM, Price J, Pereira N, et al. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol 2014;124:904-10. [Crossref] [PubMed]
- El-Nashar SA, Hopkins MR, Creedon DJ, et al. Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol 2009;113:97-106. [Crossref] [PubMed]
- Bøe Engelsen I, Woie K, Hordnes K. Transcervical endometrial resection: long-term results of 390 procedures. Acta Obstet Gynecol Scand 2006;85:82-7. [Crossref] [PubMed]
- Otsubo Y, Nishida M, Arai Y, et al. Association of uterine wall thickness with pregnancy outcome following uterine-sparing surgery for diffuse uterine adenomyosis. Aust N Z J Obstet Gynaecol 2016;56:88-91. [Crossref] [PubMed]
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest 2004;57:132-8. [Crossref] [PubMed]
- Sun AJ, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J (Engl) 2011;124:1322-6. [PubMed]
- Takeuchi H, Kitade M, Kikuchi I, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol 2006;13:150-4. [Crossref] [PubMed]
- Huang X, Huang Q, Chen S, et al. Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Womens Health 2015;15:24. [Crossref] [PubMed]
- Osada H, Silber S, Kakinuma T, et al. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online 2011;22:94-9. [Crossref] [PubMed]
- Kim TH, Lee HH, Chung SH, et al. The triple-flap method for huge uterine adenomyosis with pelvic adhesions. Reprod Biomed Online 2012;25:649. [Crossref] [PubMed]
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril 2009;92:876-85. [Crossref] [PubMed]
- Niu Z, Chen Q, Sun Y, et al. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol 2013;29:1026-30. [Crossref] [PubMed]
- Park CW, Choi MH, Yang KM, et al. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med 2016;43:169-73. [Crossref] [PubMed]
- Hou X, Xing J, Shan H, et al. The effect of adenomyosis on IVF after long or ultra-long GnRH agonist treatment. Reprod Biomed Online 2020;41:845-53. [Crossref] [PubMed]
- Chen M, Luo L, Wang Q, et al. Impact of Gonadotropin-Releasing Hormone Agonist Pre-treatment on the Cumulative Live Birth Rate in Infertile Women With Adenomyosis Treated With IVF/ICSI: A Retrospective Cohort Study. Front Endocrinol (Lausanne) 2020;11:318. [Crossref] [PubMed]
- Lan J, Wu Y, Wu Z, et al. Ultra-Long GnRH Agonist Protocol During IVF/ICSI Improves Pregnancy Outcomes in Women With Adenomyosis: A Retrospective Cohort Study. Front Endocrinol (Lausanne) 2021;12:609771. [Crossref] [PubMed]
- University College London, 2019. Modified Downregulation for Women With Adenomyosis of the Uterus Prior to Frozen-thawed Embryo Transfer. Identifier: NCT03946722. Available online: https://clinicaltrials.gov/ct2/show/NCT03 946722
- University Hospital Toulouse, 2019. National Library of Medicine (U.S.). Impact of Ultra-long Versus Long Down-regulation Protocol on IVF/ ICSI in Adenomyosis. Identifier: NCT03940807. Available online: https://clinicaltrials.gov/ct2/show/NCT03940807
- Hopital Foch. Benefit of GnRH agonist before frozen embryo transfer in patients with endometriosis and/or adenomyosis (DECATEC). 2020.
Cite this article as: Latif S, Saridogan E. Uterine adenomyoma—what we know, and what we don’t know: a narrative review. Gynecol Pelvic Med 2022;5:25.


