
Endometrial carcinoma with significant choriocarcinomatous differentiation in a nulliparous woman: a case report and literature review
Introduction
Somatic carcinoma with trophoblast differentiation or choriocarcinoma differentiation in the oesophagus, stomach, breast, ovary, bladder, lung and rectum has rarely been reported (1-7). To date, thirty-two cases of uterine carcinoma with trophoblast differentiation have been described (8-17). More than 60% of these cases (18/29) were alive with disease (AWD) or died of disease (DOD) at follow-up, suggesting that these patients had a poor prognosis and an aggressive clinical course. This study presents a case of FIGO grade 1 endometrioid adenocarcinoma with significant choriocarcinomatous differentiation with no recurrence or metastasis for 37 months after the operation. Then, the clinicopathological features and pathogenesis of this rare tumour are discussed, and treatment with surgical resection along with a combination chemotherapeutic regimen (especially for choriocarcinoma) is described. We present the following case in accordance with the CARE reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-59/rc).
Case presentation
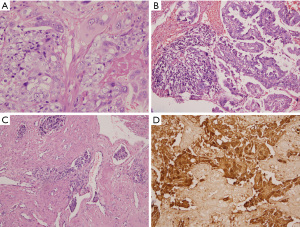
A 39-year-old gravid 0, Para 0 woman presented to a local hospital with irregular vaginal bleeding for more than 3 months on January 3, 2018. Ultrasonography showed endometrial thickening, and a slightly strong echo area measuring 6.1 cm × 4.8 cm × 4.1 cm was found in the lower uterine segment and cervix. Submucosal leiomyoma was considered, and hysteroscopic resection was performed at the local hospital on January 4, 2018. The pathology results at the local hospital showed a malignant tumour, and the patient was admitted to our hospital. Histopathology showed that 15% of the tumour was FIGO grade 1 fragmented endometrioid adenocarcinoma and that 85% of the tumour was composed of mononuclear and syncytial giant cells with hyperchromatic vesicular nuclei and prominent nucleoli, was rich in eosinophilic cytoplasm, and contained destructive growth in the myometrium, accompanied by massive haemorrhage and necrosis (Figure 1A). The two components were intermingled, and morphologically recognizable transitions could be found (Figure 1B). Obvious vascular invasion was also observed (Figure 1C). Immunohistochemical staining showed strong positive expression of ER and PR in the region of the grade 1 endometrioid adenocarcinoma and diffuse and strong positive expression of hCG in the syncytial giant cells (Figure 1D).
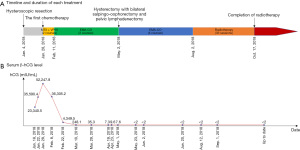
Therefore, a diagnosis of FIGO grade 1 endometrioid adenocarcinoma with choriocarcinoma differentiation was made. The highest serum β-human chorionic gonadotropin (β-hCG) concentration was 52,247.8 mIU/mL on January 28, 2018. Chest CT (January 22, 2018) showed approximately 5 scattered small nodules in both lungs ranging from 0.2 to 0.4 cm in diameter. The patient received a total of five courses of preoperative chemotherapy (1 and 2 courses of the MTX + VP16 regimen, 3 to 5 courses of the EMA-CO regimen) from January 28, 2018, to April 6, 2018, and the patient’s serum β-hCG showed a progressive decrease (52,247.8–36,305.2–4,349.5–248.1–35.3–7.3–9.6–7.6 mIU/mL). Chest CT (March 26, 2018) showed a few chronic inflammatory foci in both lungs, and no definite nodule shadow was found compared with previous chest CT. Abdominal hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy was performed on May 2, 2018. Intraoperatively, the endometrium was smooth, and there were no visible lesions in either the ovaries or fallopian tubes.
Grossly, the left wall of the uterus had thickened in an area approximately 5 cm × 4.5 cm in size. The endometrium was 0.2–0.4 cm thick and smooth. A 2 cm × 0.8 cm greyish brown region could be seen on the surface of the left ovary. There was a grey and solid nodule at the root of the left fallopian tube with a diameter of 0.4 cm. The right ovary and fallopian tube appeared normal (Figure 2A).
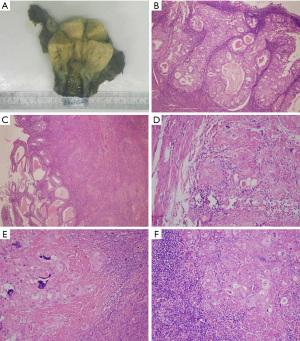
Microscopically, the FIGO grade 1 endometrioid adenocarcinoma had infiltrated less than 1/2 of the myometrium, and the surrounding endometrium showed atypical hyperplasia (Figure 2B,2C). Multiple foci of highly degenerative trophoblasts were found in the left anterior myometrium, left ovarian parenchyma, left fallopian tube nodules and pelvic lymph nodes (1/21) with extensive fibrous hyperplasia, indicating that the choriocarcinoma component had metastasized to the above sites (Figure 2D-2F).
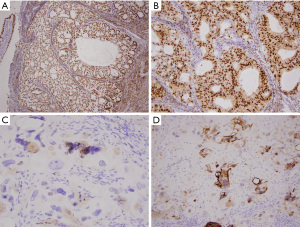
Immunohistochemically, endometrioid adenocarcinoma showed ER and PR positivity, and the highly degenerative trophoblasts showed focal mild positivity for hCG and focal expression of PLAP (Figure 3A-3D).
Postoperatively, the patient received 4 courses of chemotherapy (EMA-CO regimen) from May 17, 2018 to August 2, 2018, and 33 sessions of radiotherapy, the last of which was on October 17, 2018 (Figure 4A). Her serum β-hCG decreased to a normal level (<2 mIU/mL) 21 days after the operation and has since remained at a normal level (Figure 4B). After 37 months of postoperative follow-up, there were no signs of recurrence or distant metastasis, but abnormalities in routine liver function tests (ALT: 52–185 U/L, AST: 48–104 U/L) were found after radiotherapy and chemotherapy.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The previously reported cases were retrieved by the first author of this paper by searching the PubMed database of the United States National Library of Medicine alone using search terms that combined endometrial carcinoma with choriocarcinoma or trophoblastic neoplasia from March 1972 to November 2020. Cases deemed to be of cervical or ovarian origin were excluded.
Civantos and Rywlin were the first to report a case of endometrial cancer with trophoblast differentiation in 1972 (8). Recently, an increasing number of cases have been reported, mostly as case reports. A total of thirty-two previously reported cases of uterine carcinoma with trophoblast differentiation were reviewed in addition to the present case. These cases occur mostly in postmenopausal women; however, some cases have been reported in young women, and the outcomes appear to be unfavourable (9,15). The patients ranged in age from 33 to 88 (mean 60) years, with those older than 50 years accounting for 82% (27/33) of patients. Almost all patients presented with abnormal vaginal bleeding. In addition to this patient, a total of 7 other patients were described as nulliparous, and 1 case occurred in a virgin (10), demonstrating that the choriocarcinoma component may not be associated with pregnancy. Serum and/or urinary β-hCG elevation was found in nearly 88% (29/33) of cases during preoperative or postoperative treatment. More than 60% of cases (18/29) were AWD or DOD at follow-up, suggesting that these patients had a poor prognosis and an aggressive clinical course.
The most frequent histological subtype of endometrial carcinoma is endometrioid adenocarcinoma (11/33), with follow-up results reported for 9 cases and the proportion of total/dead patients being 5/2 for G1, 1/0 for G2 and 3/1 for G3. Compared with the proportions for simple endometrioid endometrial carcinoma (G1, 408/19; G2, 260/23; and G3, 42/8) reported by Khatib et al. (18), it is speculated that endometrial carcinoma with trophoblast differentiation, particularly G1, may have a poor prognosis. However, because of its rarity, more cases are needed to increase the reliability of the results. The most frequent histological subtype of the trophoblastic component is choriocarcinoma (28/33). There were significant differences in the proportion of trophoblastic components: the smallest was less than 1 mm in size (11), and the largest was 90% (16). Our case, a young, nulliparous patient with significant choriocarcinomatous differentiation, is one of the few reported cases with a relatively favourable prognosis.
Due to the rarity of this disease, the pathogenesis of endometrial carcinoma with trophoblast differentiation remains unclear. Whether trophoblastic tumours are primary tumours or evolve based on endometrial cancer also remains debated. A molecular comparison of the two components conducted by Olson et al. (19) showed the same clonal origin and found evidence of evolution from an endometrioid carcinoma to a trophoblastic tumour. Neumann et al. (17) compared the molecular genetic sequence changes between blood and choriocarcinoma components and found that the choriocarcinoma component was most likely non-gestational. Acosta et al. (20) found that both endometrial carcinoma and trophoblastic components had an almost identical mutational profile, and the presence of trophoblastic tumour components appeared to correlate with underlying genomic instability. In our case, there was no history of menopause and no known pregnancy, and the transition between the two components was observed microscopically, suggesting that the choriocarcinoma component is close to the endometrioid carcinoma and most likely non-gestational in nature.
The prognosis of previously reported cases is extremely poor, with a few exceptions. Yamada et al. (12) reported a case of poorly differentiated endometrioid adenocarcinoma with a choriocarcinomatous component (50%), which had the best prognosis of all reported cases, namely, a survival time of 50 months since surgery. However, the above patient experienced recurrence and metastasis of the choriocarcinoma component 9 months after the operation and 5 cycles of chemotherapy for the CTP regimen at the vaginal wall. Then, the chemotherapy regimen was switched to etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine (EMA-CO), which resulted in a good prognosis. Interestingly, in our case, the preoperative imaging findings revealed that pulmonary metastasis had likely occurred, and postoperative metastasis was also found in the left ovary, fallopian tube and pelvic lymph nodes. After surgical resection, chemotherapy (mainly the EMA-CO regimen) and radiotherapy, a relatively good prognosis was obtained. Analysis of the treatment of the above 2 cases shows that surgical resection with a combination chemotherapeutic regimen is effective for some patients even when metastasis has been detected. However, there were only a few “exceptions” in more than 30 reported cases, as many patients were relatively insensitive to chemotherapy. It is speculated that this may be related to the proportion of trophoblast components, individual sensitivities and the response to chemotherapy, the histological subtype of endometrial carcinoma and stage at presentation, or other unknown factors. The number of reported cases is too small for us to determine the most effective treatment. Because cases of endometrial carcinoma with trophoblast differentiation show a highly aggressive clinical course, characterized by early recurrence and metastasis and a poor response to therapy, the treatment options mentioned above may still be worth trying. Serum β-hCG levels can help us monitor the existence of choriocarcinoma components and patient prognosis.
After 37 months of follow-up, imaging examination showed no clear space occupation, and the serum β-hCG level was stable at a normal level, which was quite remarkable. Surgical resection was performed in almost all previously reported cases; however, further chemotherapy was not administered to some patients after the operation, especially in cases with earlier clinical stages or in the absence of discovery of trophoblast components, and tumour recurrence or metastasis to other organs was evident soon after (14). Therefore, it is important to identify trophoblast components in time. Is it more important to suppress the choriocarcinoma component first when the endometrial carcinoma situation is relatively stable? Although studies have shown that choriocarcinoma components may originate from somatic cells, these components still have the characteristics of choriocarcinoma (such as hormone secretion), so the chemotherapy regimen for choriocarcinoma seems to be effective at inhibiting the development of this rare entity. Limited by the small number of cases, the above questions have not been completely addressed; more cases and studies are needed to answer these questions.
In summary, we present a case of endometrioid adenocarcinoma with extensive choriocarcinoma differentiation in a nulliparous young woman; this is one of the few cases reported with a relatively favourable prognosis after surgery combined with chemotherapy, particularly for choriocarcinoma. However, similar situations are reported less frequently, and more data are needed.
Overall, cases with trophoblast differentiation are rare, and most of them show extremely malignant biological behaviour and poor prognosis. Therefore, it is important to identify trophoblast components in time, as this may affect the choice of treatment and prognosis.
Acknowledgments
The authors thank Dr. Zhirong Du and MS Laura for editing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-59/rc
Peer Review File: Available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-21-59/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McKechnie JC, Fechner RE. Choriocarcinoma and adenocarcinoma of the esophagus with gonadotropin secretion. Cancer 1971;27:694-702. [Crossref] [PubMed]
- Liu AY, Chan WY, Ng EK, et al. Gastric choriocarcinoma shows characteristics of adenocarcinoma and gestational choriocarcinoma: a comparative genomic hybridization and fluorescence in situ hybridization study. Diagn Mol Pathol 2001;10:161-5. [Crossref] [PubMed]
- Erhan Y, Ozdemir N, Zekioglu O, et al. Breast carcinomas with choriocarcinomatous features: case reports and review of the literature. Breast J 2002;8:244-8. [Crossref] [PubMed]
- Oliva E, Andrada E, Pezzica E, et al. Ovarian carcinomas with choriocarcinomatous differentiation. Cancer 1993;72:2441-6. [Crossref] [PubMed]
- Obe JA, Rosen N, Koss LG. Primary choriocarcinoma of the urinary bladder. Report of a case with probable epithelial origin. Cancer 1983;52:1405-9. [Crossref] [PubMed]
- Yamamoto S, Tanaka H, Takeo H, et al. Primary pulmonary choriocarcinoma combined with adenocarcinoma. Pathol Int 2006;56:402-7. [Crossref] [PubMed]
- Verbeek W, Schulten HJ, Sperling M, et al. Rectal adenocarcinoma with choriocarcinomatous differentiation: clinical and genetic aspects. Hum Pathol 2004;35:1427-30. [Crossref] [PubMed]
- Civantos F, Rywlin AM. Carcinomas with trophoblastic differentiation and secretion of chorionic gonadotrophins. Cancer 1972;29:789-98. [Crossref] [PubMed]
- Nguyen CP, Levi AW, Montz FJ, et al. Coexistent choriocarcinoma and malignant mixed mesodermal tumor of the uterus. Gynecol Oncol 2000;79:499-503. [Crossref] [PubMed]
- Kalir T, Seijo L, Deligdisch L, et al. Endometrial adenocarcinoma with choriocarcinomatous differentiation in an elderly virginal woman. Int J Gynecol Pathol 1995;14:266-9. [Crossref] [PubMed]
- Carta G, Accurti V, Di Nicola M, et al. Uterine endometrioid carcinoma with focal area of choriocarcinomatous differentiation: case report. Eur J Gynaecol Oncol 2014;35:731-3. [PubMed]
- Yamada T, Mori H, Kanemura M, et al. Endometrial carcinoma with choriocarcinomatous differentiation: a case report and review of the literature. Gynecol Oncol 2009;113:291-4. [Crossref] [PubMed]
- Brouwer WK, van Raalte HA, van Veelen H, et al. An extra gonadal non-gestational choriocarcinoma in a woman treated for an endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol 1995;60:195-9. [Crossref] [PubMed]
- Rawish KR, Buza N, Zheng W, et al. Endometrial Carcinoma With Trophoblastic Components: Clinicopathologic Analysis of a Rare Entity. Int J Gynecol Pathol 2018;37:174-90. [Crossref] [PubMed]
- Cai H, Zhou R, Liang W, et al. Dedifferentiated endometrioid adenocarcinoma with trophoblastic components and elevated serum alfa-fetoprotein: A case report and literature review. Medicine (Baltimore) 2018;97:e0551. [Crossref] [PubMed]
- Yadav S, Sagar N, Mallya V, et al. Extensive trophoblastic differentiation in case of an endometrial carcinoma. Indian J Pathol Microbiol 2018;61:614-6. [Crossref] [PubMed]
- Neumann G, Brasch-Andersen C, Fagerberg C, et al. Endometrioid adenocarcinoma with a co-existing non-gestational choriocarcinoma in uterus. Ugeskr Laeger 2019;181:V03190167. [PubMed]
- Khatib G, Gulec UK, Guzel AB, et al. Prognosis Trend of Grade 2 Endometrioid Endometrial Carcinoma: Toward Grade 1 or 3? Pathol Oncol Res 2020;26:2351-6. [Crossref] [PubMed]
- Olson MT, Gocke CD, Giuntoli RL 2nd, et al. Evolution of a trophoblastic tumor from an endometrioid carcinoma--a morphological and molecular analysis. Int J Gynecol Pathol 2011;30:117-20. [Crossref] [PubMed]
- Acosta AM, Sholl LM, Cin PD, et al. Malignant tumours of the uterus and ovaries with Mullerian and germ cell or trophoblastic components have a somatic origin and are characterised by genomic instability. Histopathology 2020;77:788-97. [Crossref] [PubMed]
Cite this article as: Xie Y, Li Q, Yang K, Liu T, Liu J, Li L. Endometrial carcinoma with significant choriocarcinomatous differentiation in a nulliparous woman: a case report and literature review. Gynecol Pelvic Med 2022;5:29.


