
Role of immunotherapy in ovarian cancer: a narrative review
Introduction
Ideally, the immune system can identify and eliminate cancer cells. However, many tumour cells evade the host immune system and survive in chronically inflamed microenvironment, leading to disease progression (1-3). Exploiting host immunity for the benefit of the patient through the induction, enhancement, and suppression of self-immunity, is the goal of cancer immunotherapies. Despite successes of immune checkpoint inhibitors (ICI) (the 2018 Nobel Prize-winning treatment) targeting cytotoxic T lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) that normally guard against autoimmunity, overall, response to immunotherapies varies across different tumours with limited efficacy (4,5). Therefore, there is a need to characterise the tumour-immune functioning in other cancers to develop appropriate treatments.
The concept of “hot” and “cold” tumours differentiates solid tumours that are vulnerable to cancer immunotherapies. Hot tumours, such as melanomas, are inflamed by infiltrating T lymphocytes and pro-inflammatory cytokines which makes them more susceptible to ICIs, while cold tumours are not (6).
Presumably, ovarian cancer (OC), the deadliest gynaecological cancer accounting for over 200,000 deaths worldwide in 2020, would also benefit from immunotherapies (7). Due to non-specific symptoms and a lack of specific and sensitive biomarkers, diagnosis is often at late stages (III-IV) with a 29% relative 5-year survival rate compared to stage I (92%) (8). Over 90% of OC tumours are epithelial in origin, with the high grade serous (HGSOC) histotype being the most common (70%) (8). Epithelial tumours are associated with a high relapse rate, which occurs in 70–80% of patients (9). Additionally, tumours are both genetically and non-genetically heterogeneous, which contributes to differential responses to treatments.
Tumour T lymphocyte infiltration is associated with a more favourable prognosis in OC (10-12). A recent prospective survival cohort study of over 5,500 patients revealed longer overall survival (OS) (~2.3 years) in HGSOC patients with tumour infiltrating T lymphocytes (TILs) compared to those without TILs (13). Metastasis is most frequent to the adipose tissue of the omentum, which is characterised by highly vascularised immune structures known as “milky spots” (14,15). Currently, one theory of relapse is being attributed to cellular dormancy, a proposed novel hallmark of cancer where cells are capable of undergoing G0 cell cycle arrest, attain chemo-resistant mechanisms, and evade immunity (16). Immune evasion mechanisms promote metastasis and may be particularly important in concealing a small subset of residual tumour cells that avoid host rejection to eventually proliferate. This review aims to outline various immune evasion strategies and advances in OC immunotherapies.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-18/rc).
Methods
A literature search (Table 1) was conducted on PubMed utilising the keywords “ovarian cancer”, “epithelial ovarian cancer”, “immunotherapy”, “immune evasion”, and “relapse” in various combinations. “Immunotherapy” was defined as therapies that modulate a person’s immune system to enhance or suppress its action against cancer, therefore, all other therapies were excluded. This review primarily prioritised papers from the last 10 years (from 2010 onwards). However, being a narrative review, this paper also acknowledges earlier well-cited papers critical to OC treatment development. The primary literature search was supplemented by citing and performing secondary searches based off papers found in reference lists, as well as comparisons to other types of cancers. All types of papers in English were considered, including abstracts. Themes from the literature review were organised into subheadings of this review.
Table 1
| Items | Specification |
|---|---|
| Date of search | 2020 October 2 & 2021 June 11 |
| Databases and other sources searched | PubMed |
| Search terms used | “ovarian cancer”, “epithelial ovarian cancer”, “immunotherapy”, “immune evasion”, “relapse” |
| Timeframe | 2010–2021 |
| Inclusion and exclusion criteria | English, all study types are included |
| Selection process | Themes and relevant papers were identified, resulting in secondary searches and other citations from reference lists. Topics were proposed by first author and agreement obtained from all authors |
Immune evasion strategies
The evasion of immune recognition leads to the loss of tumour rejection. Numerous barriers limit infiltrating T cells’ action on tumours including (Figure 1): immunosuppressive immune infiltrate, suppressive molecules, lack of co-stimulation, aberrant tumour vasculature, hostile environment, suppressive receptors, inhibitory enzymes, and chemokine network that attracts immunosuppressive cells (17). Low immunogenicity is classically attributed to the downregulation of the human leukocyte antigen (HLA) class I and II peptides that present endogenous antigens to cytotoxic T lymphocytes (18). High ovarian tumour HLA expression is correlated with 19 months longer OS (19). Tumours may also overexpress immunoglobulin CD47, which, in complex with signal-regulatory protein α (SIRPα), acts as a marker of “self” and inhibits macrophage phagocytosis (20,21). Ovarian tumours with high CD47 expression have been associated with poor clinical prognosis (22). Furthermore, antibodies targeting cell surface proteins (antigens) may be endocytosed and degraded within lysosomes (23), thus directly regulating antigen modulation and immunogenicity.
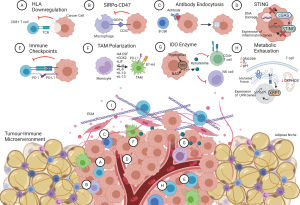
A novel area for cancer research is the defective stimulator of interferon genes (STING) signalling. STING, a transmembrane protein, normally resides within the endoplasmic reticulum (ER) and dimerises to translocate to the cytosol in response to cytosolic DNA (foreign or DNA-damage) (24). It is part of the innate immune system and is important in autophagy and anti-tumour immune responses, particularly senescence or activation of immune defence mechanisms (25). The cGAS-cGAMP-STING pathway stimulates the type I interferon (IFN) production at early stages, while autocrine/paracrine JAK-stimulated STAT1 later to activate host immune responses (26-28).
In HGSOC cell lines, STING signalling was found to be downregulated epigenetically and exhibited loss of NFκB signalling, responsible for type I IFN production (24). Further, the deubiquitylase USP35 downregulates STING (29). USP35 overexpression has been correlated with a “cold” tumour state (29). In a murine model, combining both STING agonists and PD-1 inhibitors was associated with reduction in tumour burden and ascites accumulation, higher antigen presentation and increased IFN responses (30). Oncolytic viruses (i.e., herpes simplex) could also be used as therapeutic means as cells with defective STING signalling are more susceptible to infection (24). Thus, loss of STING signalling in response to cytosolic DNA prevents cytokine production that triggers immune responses.
Tumour-induced and micro-environmental factors can suppress immune reactivity. Tumour-induced immunosuppression mainly involves immune checkpoints, which are crucial in maintaining self-tolerance. Research has primarily focussed on PD-1 and CTLA-4 checkpoints. However, the function of emerging ones such as LAG-3, TIGIT, VISTA, TIM-3, B7-H3, Singlec-15 and BTLA should also be characterised in HGSOC (31-37). Tumours can induce cytotoxic T lymphocyte anergy and exhaustion through checkpoints. Programmed cell death ligand 1 (PD-L1), involved in peripheral tolerance, is expressed by tumours to deactivate T lymphocyte cytotoxicity and inhibit the cytotoxic IFN signalling cascade (38). Thus, immune checkpoints are immunomodulatory. Further, tumours are involved in cell-mediate cross-talk by secreting various cytokines and excretory vesicles such as exosomes [carrying i.e., Fas ligand (FasL)] to promote anti-tumour responses (39).
Immunosuppressive tumour-immune microenvironment (TIME)
The TIME involves various immune cells that work to eliminate the tumour and immunomodulate the response, including antibody-secreting B lymphocytes, T lymphocytes, FoxP3+ T regulatory lymphocytes (HGSOC only), dendritic cells (including plasmacytoid), mast cells, myeloid-derived suppressor cells (MDSC), tumour associated macrophages (TAM), and natural killer cells (NK) (40,41). As a complex physical structure composed of the extracellular matrix, blood vessels, fibroblasts, immune cells and surrounding signalling molecules, it can act as a barrier to immune infiltration (42).
Tumours may be capable of transforming TILs to influence anti-tumour immunity. A previous study has shown that CD8+ T-lymphocytes cultured with SKOv3 cell lines were transformed into functioning CD8+ regulatory T cells (Tregs) (43). Similarly, the presence of interleukin (IL)-8, overexpressed within the tumour and ascites, can recruit and induce Jagged2 expression in neutrophils (44,45). Jagged2 is part of the Notch pathway and directly negatively regulates CD8+ T cell effector molecules such as granzyme B and IFN-γ (44). Furthermore, tumours can influence the microenvironment. The intracellular enzyme idoleamine-2,3-dioxygenase 1 (IDO-1), the rate limiting step in tryptophan (essential amino acid) metabolism, is expressed by tumours (46). IDO-1 produced by tumours or competent dendritic cells can immunosuppress CD8+ T-lymphocytes, CD4+ Th1 cells and NK cells (47). Kynurenine is a toxic metabolite of tryptophan and is released into the microenvironment. As an aryl hydrocarbon receptor ligand, kynurenine is transported into CD8+ and CD4+ T-lymphocytes and promotes activated CD4+ T cells into immunosuppressive Foxp3+ Tregs (48,49). IDO-1 is also expressed within another cell type in the TIME, TAMs.
TAMs are macrophages functioning within the TIME polarised to the immunosuppressive M2-like phenotype through prostaglandin E2, IL-6, IL-10, leukaemia inhibitory factor (LIF), cyclo-oxygenase 2 (COX2), colony stimulating factor-1 (CSF-1), and STAT6 signalling (IL-4, IL-13) (50-53). The M2 subtype is associated with poor prognosis in HGSOC, as higher M1/M2 ratios were correlated with increased progression-free interval (PFI), progression-free survival (PFS) and OS (54). Recently, our group has shown that the epithelial-mesenchymal transition (EMT)-high HGSOCs, a subtype known to be strongly correlated with poor prognosis, are associated with an enrichment in M2 macrophages (55). TAMs produce anti-inflammatory cytokines TGF-β, IL-10 and IL-13, as well as epidermal growth factor (EGF) associated with spheroid formation in transcoelomic metastasis (56). Furthermore, TAMs secrete immunosuppressive chemokines such as CCL18 and CCL22 to drive Tregs (57,58), and suppress T-cell immunity through highly expressed surface molecules PD-L1 and B7-H4 (59,60). Thus, TAMs are a further barrier to immune treatment strategies.
Cancer stem cells (CSCs), a cellular population capable of self-regeneration and differentiation, is present within the microenvironment. These cells have been implicated in metastasis, tumorigenicity, and relapse (61). They can repopulate a heterogenous tumour similar to the primary tumour and are chemo resistant (62), thus suggesting the ability to evade the immune system. Markers of ovarian CSCs include aldehyde dehydrogenase 1 (ALDH1A1), CD24+, CD44+, CD117+, CD133+ and epithelial cellular adhesion molecule (EpCAM). In addition, these cells exhibit pluripotency factors and established stem cell pathways including Notch, Hedgehog, mTOR, Wnt, and STAT3 (63). Ovarian CSCs influence the vascular tumour microenvironment, secrete pro-inflammatory cytokines and are associated with M2 macrophage polarization (64,65). However, they are difficult to target as they comprise approximately 1% of a tumour (66). In OC, CSCs have not been isolated from patient tumours and directly tested in immunocompromised mice for tumour-initiating potential; most studies passage cells from patient tumours or use cell lines, which are associated with genetic and phenotypic alterations (67).
Cellular metabolism
The metabolic state of immune cells is necessary for optimal host immunity functioning, however, can be altered by the tumour microenvironment. ER stress is a process where stressors such as chemotherapy, lactic acidosis, hypoxic conditions and glucose deprivation may lead to unfolded proteins within the ER and the unfolded protein response (UPR) (68). The tumour microenvironment can induce UPR in intra-tumoural CD4+ T-lymphocytes through IRE1α-XBP1 (69).
Within the ascites environment, limited glucose availability reduces glucose transporter GLUT1 expression, reduces glycolysis and impairs N-linked protein glycosylation, leading to ER stress. The activation and production of XBP1 downregulates glutamine transporters into the mitochondria to hamper the citric acid cycle and oxidative phosphorylation (69). Subsequently, this leads to reduced T lymphocyte function. Similarly, previous research has identified that dendritic cells, those linking innate and adaptive immunity, are suppressed metabolically. In dendritic cells, lipid peroxidation by-products from reactive oxygen species (ROS) triggers ER stress, UPR, XBP1 activation and induces triglyceride biosynthesis (70). This led to lipid accumulation within dendritic cells and reduced antigen presentation to anti-tumour T-lymphocytes. Thus, the UPR is suggested to act as an “immuno-metabolic checkpoint” (69). In other cancers, increased fatty acid levels have also been implicated in immuno-suppressive effects of polymorphonuclear-MDSC and macrophages (71). Veglia et al. [2019] have shown that combining fatty acid transport protein 2 (FATP2) inhibitors with ICIs in mice arrests tumour progression. Thus, altered metabolic state can influence pro-tumour responses, lead to T-cell exhaustion and should be considered when developing treatment strategies.
Despite TILs being associated with a more favourable prognosis, tumour microenvironment has evolved mechanisms to overcome host immunity. While augmenting TILs may overcome these mechanisms, treatments may only lead to immuno-editing that enhance immune-resistant tumour cells. It should also be noted that established evidence shows that tumorigenic cells can covert between states of plasticity (72,73), whose implications for immunotherapies (Figure 2) are unclear.
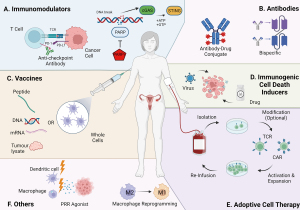
Immunotherapies
There is an urgent need for novel therapies for HGSOC. Efficacy of first line treatment is limited as the majority of women relapse, including those who initially respond well to treatment (9). Standard treatment since the late 1970s involves primary debulking surgery (PDS) followed by platinum-based chemotherapy (74). The drug regimen includes carboplatin, a cross-linking purine DNA agent, and paclitaxel, a microtubule stabilizer which arrests cells in the G2/M phase of the cell cycle (75,76). These drugs mainly target highly proliferating cells, which is problematic considering residual cells post-chemotherapy are thought to be dormant. More recently, poly-ADP polymerase inhibitors (PARPi) such as olaparib and niraparib, are becoming part of the standard management of ovarian tumours as maintenance therapy (77,78).
Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) has been suggested for advanced stage HGSOC patients (IIIC to IV) to decrease tumour burden prior to surgery (79). However, the mechanism of action of these drugs does not change with changing the order of treatment. Accordingly, NACT fails to affect immunosuppressive mechanisms despite increasing T-lymphocyte responses (80).
Targeted antibodies
Antibodies are proteins produced by B lymphocytes in response to antigens. To date, only one antibody, bevacizumab, has been approved by the Food and Drug Administration (FDA) for treating OC. However, it does not qualify under the definition of an “immunotherapy”. Antibodies as immunotherapies can be utilised as drug delivery systems, known as antibody-drug conjugates (ADC). These systems are combinations of chemotherapy and immunotherapy, composed of a monoclonal antibody joined to a cytotoxic payload (drug) using a synthetic chemical linker. The antibody is highly specific to antigens on target cells, with immunoglobulin G (IgG) being the most common antibody available in four isotypes (81). The linker can either be cleavable or non-cleavable. Cleavable linkers release the drug extracellularly or intracellularly by specific proteases or pH ranges, while non-cleavable ones only intracellularly after complete degradation of the antibody within the target cell’s lysosome (82). The chemotherapeutic agents attached as payloads are designed to be highly potent to induce cytotoxicity at the minimum effective dose, have low immunogenicity, long half-life and low molecular weight (83).
ADCs have been FDA-approved for the treatment of Hodgkin’s lymphoma, anaplastic large cell lymphoma, acute myeloid leukaemia, B-Cell acute lymphoblastic leukaemia and breast cancer. In OC, the only ADC evaluated in phase III trials is mirvetuximab soravtansine, which targets folate receptor alpha (FRα)-positive cells. The results were underwhelming, with no significant difference in PFS compared to a choice of paclitaxel, topotecan or pegylated liposomal doxorubicin (84,85). Further, mirvetuximab soravtansine exhibits the bystander effect, where cytotoxic activity of the ADC can be extended to nearby cells (86). This can be beneficial for heterogeneous tumours; however, it may affect antigen-negative tissue nearby as well. Targets to other antigens in preclinical settings and in early clinical studies have included tissue factor, mesothelin, NaPi2B, Trop2, and MUC16 (CA125), all with variable levels of success (81). Newer studies are now examining ADCs in combination with PARPi for BRCA mutant patients, and in combination with multiple cancer immunotherapies such as PD-1/PD-L1 antibodies, Ox40 ligand and GITR ligand fusion proteins, which produced synergistic responses (87,88). However, ADC technology is quite sophisticated and is subjected to many factors that influence its success, including ADC structure, payload type, pharmacokinetic, antigen heterogeneity/masking, and intra-tumoural factors such as drug efflux, cytoskeleton or lysosome proteolytic activity abnormalities (89). Thus, future investigations in this area could be challenging and subjected to inter-patient variability.
Upcoming antibody treatments include bispecific antibodies, which contain two antigen-binding sites. The first bispecific antibody, catumaxomab, was approved in 2009 by the European Medicines Agency for the intraperitoneal treatment of ascites and had shown reduced tumour burden and ascites in OC. However, it was withdrawn from the market for financial reasons in 2017. Catumaxomab was known as a “trifunctional” bispecific antibody, as it consisted of rat and mouse antibody chains targeting EpCAM/CD3, and a fragment crystallizing region that bound to either macrophages, NK cells or dendritic cells (90). A more novel EpCAM/CD3 targeting drug is solitomab, a bispecific T cell engager (BiTE). BiTEs are a class of bispecific antibodies composed by two single-chain variable fragments (scFv) linked by a peptide chain rather than a stem. Solitomab has only been tested in vitro, where chemotherapy-resistant cells were sensitized to cytotoxic T-cells, and ex vivo, where malignant ascites had decreased tumour cells and increased cytotoxic T-cell markers compared to control (91). BiTEs bind to a cell-surface molecule (i.e., CD3) on a T cell, and to a tumour marker to induce polyclonal T cell expansion. Currently, the only approved BiTE binding to CD3 is blinatumomab, used in the treatment of chemotherapy-refractory acute lymphoblastic B cell leukaemia (92).
Current clinical trials on bispecific antibodies in OC are in phase 1 or 2 stages and recruiting for treatments targeting MUC16/CD3 (NCT03564340), PD-L1/CD27 (NCT04440943), CTLA-4/LAG-3 (NCT03849469), PD-1/CTLA-4 (NCT03517488), EGFR/TGF-β (NCT04429542), as well as the recently completed DLL4/VEGF (NCT03030287). Limitations of bispecific antibodies, particularly BiTEs, include reduced serum half-lives and the difficulty of predicting T-cell profiles within the TIME (93). Further, they may be limited by T-cell exhaustion. Overall, bispecific antibodies have the potential of mass production and “off the shelf” T-cell therapy given intravenously, which overcome the individualised approach of CAR T-cells. Other antibody developments, such as trispecific, which have an additional T-cell protein binding site that prolongs T-cell activity against a tumour, are yet to be tested in OC. Further studies are warranted to develop antibodies against OC.
OC vaccines
The goal of cancer vaccine therapy is to induce immune responses against specific malignant cells using tumour associated antigens (TAA) and generate specific effector T-lymphocytes against tumours. Vaccines have been made from a variety of sources, including DNA, mRNA, cells, proteins, bacteria, viruses, and other small molecules. Vaccines can be either prophylactic or therapeutic. Prophylactic vaccines are developed to prevent and reduce cancer incidence, morbidity and mortality, while therapeutic ones treat already existing malignancy (94). Thus far, no vaccines have been approved for clinical use in OC. Targetable TAAs examined have included: overexpressed antigens, cell surface proteins in higher quantities on cancer cells than normal cells; tissue-specific TAAs, antigens common to both the tumour and the tumour’s tissue-of-origin; and cancer-testis antigens, TAAs normally present in male germline cells (95). Although cancer-testis antigens such as OY-TES-1, MAGE-A1, MAGE-A4 and MAGE-C1 have been found to be shared among 95% of OC tumours, not one is common to all tumours (96). Similarly, the highly studied NY-ESO-1 antigen, correlated with a more aggressive phenotype, is expressed in approximately 41% of tumours (97,98). Therefore, inter and intra-tumour antigen heterogeneity is a limiting factor of vaccines and no single OC-specific immune target exists (99). Other previous vaccine strategies including protein/peptide-based vaccines and recombinant viral vectors, expressing multiple cancer antigens, have shown some anti-tumour efficacy and increased immune responses, but have not made it far yet in clinical trials.
A novel area of research revolves around personalised vaccines, which stem from deep sequencing studies that discovered neo-antigens (NeoAgs). These antigens arise from somatic mutations within tumours that result in novel peptides absent from the human genome (100,101), thus entirely cancer-specific and unlikely to induce tolerance. NeoAgs early preclinical evidence had suggested that OC’s low mutational tumour burden may hinder such vaccinations (102), however, whole-exome sequencing and transcriptomics studies have identified their existence in OC (103,104). Greater NeoAgs burden in pre-chemotherapy samples and greater CD8+ T-lymphocyte infiltrate were independently associated with increased survival, while no correlations were found between NeoAgs and CD8+ infiltrate (105). Furthermore, relapsed tumour samples exhibited 78% more NeoAgs expressions than untreated primary samples, of which a mean 5% was a chemotherapeutic contribution (105). This has implications for targeting tumorigenic cells with greater specificity without inducing toxicity to other tissues, although these somatic mutations are rare events within individual patients (106).
Dendritic cells are the most promising type of vaccine treatment due to their roles in innate and adaptive immune systems. TAAs are commonly presented to dendritic cells ex vivo by whole cell lysate and tumour-associated peptides, allowing a wide spectrum of patient-specific NeoAgs and TAAs to be targeted. Loading dendritic cells with antigens using hypochlorous acid oxidation induces stronger T-cell responses than freeze-thaw processing and UVB irradiation methods (107,108). A promising personalised dendritic cell vaccine was created using autologous tumour lysate and tested in combination with bevacizumab. Results suggested that OC patients had higher (78%) OS at 2 years compared to no vaccine (44%) (109). Similarly, a dendritic vaccine pulsed with autologous tumour cell lysate (DCVAC/OvCA vaccine) was tested in the SOV02 Phase II trial. Interestingly, no significant difference was identified for PFS, which is where most current clinical trials find statistical significance, but in the OS. Patients treated with the DCVAC/OvCa vaccine exhibited a median OS of 35.5 months compared to 22.1 months in patients undergoing carboplatin with gemcitabine treatment (110). This vaccine will further be tested in the phase III VITALIA trial (NCT03905902). Despite the potential, studies are limited by low sample sizing, labour-intensive protocols requiring surgery to retrieve tumours and having sufficient tumour lysate available to utilize in a vaccine.
Adoptive cell therapy (ACT)
ACT involves extracting autologous immune cells (apheresis) to expand and modify them ex vivo, then reinfusing back into the patient to combat the tumour. Strategies thus far in OC have focussed on two cell types: MHC-independent, and MHC-dependent. MHC-independent includes lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, NK cells and chimeric-antigen receptor (CAR) T-cells. Alternatively, MHC-dependent cells are tumour-infiltrating lymphocytes (TILs) and T cell receptor (TCR) T cells. CAR T-cell based therapies are the more widely studied ACT since this approach has been successful in haematological cancers, which has laid foundations for other T cell therapies, including TCR T-cells.
TILs are blood lymphocytes (CD4+, CD8+ T cells, B cells and NK cells) that identify and infiltrate tumours independently. Their presence within tumours is associated with increased patient survival in many solid tumours, including breast and OCs (111,112). Not all T lymphocyte infiltrations are beneficial, though, such as Treg cells which inhibit cytotoxic CD8+ T lymphocytes and are associated with poor prognosis (11). A major advantage of TILs is that they are detectable for many years after infusions (113), and likely to detect tumour recurrences before seen on scans. However, clinical trials testing TILs in OC in the ‘90s, mainly T lymphocytes, had conflicting results between trials and high-dose IL-2 toxicity (114-116). It is suggested that many variables may influence the efficacy of TILs, including immune checkpoints, IDO, high COX expression, NKG2D receptor ligands, proinflammatory cytokines and immuno-suppressive cells within the TIME (117). Further, approximately 10% of infiltrating CD8+ T cells can recognise autologous tumour, suggesting that infiltration of tumours does not imply anti-tumour activity and may only be bystander cells acting as effector cells (118). Currently, it is suggested that TILs in conjunction with ICIs, decreasing IL-2 patient toxicity and engineering modifications may increase in vitro cell expansion and efficacy in patients (119).
TCR T- and CAR T-cells both undergo ex vivo modifications of their receptors to target tumorigenic cells. TCRs are composed of heterodimer TCRα and TCRβ chains that recognise intracellular antigens presented by MHC class molecules, thus requiring haplotyping to avoid graft-versus-host disease (GvHD). This is a life-threatening autoimmune condition, where the donated (graft) immune cells view the recipient’s body as foreign and attack it. Generally, high-affinity TCR T-cells are subject to central and peripheral tolerance, thus naturally occurring TCRs targeting tumour antigens have lower-affinity (120). Studies have previously focussed on antigens MAGE-A4, WT1 and NY-ESO-1, and, more recently, on developing TCR T-cells targeting NeoAgs, since T-cells targeting NeoAgs can infiltrate tumours (103,121). These cells can withstand central tolerance, thus implying prolonged anti-tumour responses, and T-cell responses have been found to be associated with higher mutation burdens and NeoAgs loads (104). Although not all NeoAgs are immunogenic. As comprehensive screens for T-cell responses to NeoAgs have had a validation rate of 0.5–2%, current strategies are focussing on improving validation and timing of protocols (i.e., 2 weeks) (104,122). It may be more effective to prioritise NeoAgs in vaccines rather than in TCR T cells for logistical reasons.
Alternatively, CAR T-cells utilise an external scFv to recognise external antigens (TAAs) on tumorigenic cells. They have evolved through four generations of receptors, including the addition of a costimulatory domain CD28/4-1BB/OX-40 (2nd generation), two or more costimulatory domains (3rd generation), and constitutively secreting/inducible transgenic IL-12 cytokine cassette to remodel the tumour microenvironment (4th generation). Recently, a preclinical model was used to examine the effects of CAR T-cells constitutively expressing IL-12 on the tumour microenvironment. These CAR T-cells retained efficacy when exposed to PD-L1 and depleted TAMs using Fas/FasL (123). Depleting M2 macrophages or converting them into M1 (inflammatory) macrophages is one strategy to decrease the immuno-suppression of the TIME. Further, the T cells retained their cytotoxicity, proliferation and underwent less apoptosis than CAR T-cells without IL-12. However, this study was done in the ascites environment, which appears at late stages and allows easier 3D access to tumorigenic cells than the TIME. CARs have the ability of recognising tumour antigens independent of MHC molecules, thus are not affected by immune evasion strategies such as HLA downregulation. However, CARs are created to recognise only common tumour specific antigens, but the recognition of patient-specific ones would likely be more effective for treatment strategies (124). Most of the antigens have been studies in preclinical models and few are currently being evaluated in phase I and II clinical trials such as mesothelin, HER2 and FRα (124). CAR T-cells have targeted CSCs through EpCAM (125,126). The studies showed anti-tumour activity in cell lines and immunodeficient mice, but need to go through clinical trials. Surprisingly, no other CSC markers have been targeted by CAR T-cells in OC.
ACT depends on the tumour infiltrating ability of immune cells, which is associated with challenges due to the immunosuppressive TIME. Further, a potentially life-threatening condition known as cytokine release syndrome (CRS) causing acute inflammation is associated with elevated cytokine IL-6 levels (127). This condition is caused by in vivo multiplication of CAR T-cells and characterised by increased levels of acute-phase proteins, high fever, respiratory and cardiovascular insufficiency and neurotoxicity (127). Finally, not all T cell targets are common to tumours, and may be found in other areas of a patient’s body. This is known as “on-target, off tumour” toxicity, which has led to modifications such as a chimeric costimulatory receptor, trans-signalling T cells (two distinct CARs), suicide genes, and oxygen-sensitive CAR scaffolds (128-131), to overcome this significant limitation. Thus far, ACT has not been optimised to withstand the tumour microenvironment and the negative metabolic cues and therefore, further bioengineering optimisation is required.
To overcome the safety challenges of T cells, studies are now examining alternative effector immune cells, NK cells. In OC, NK cells have been shown to co-infiltrate tumours with CD8+CD103+ T cells, and a higher percentage of ascites-derived NK cells within a lymphocyte fraction has been associated with increased OS (132,133). These cells can be derived from peripheral blood, umbilical cord blood, induced pluripotent stem cells (iPSC), and irradiated NK-92 cell lines, although they compose a minority (10–15%) of peripheral blood lymphocytes (134,135). NK cells are not HLA-dependent and do not need prior sensitisation for action, thus their effect depends on the presence of inhibitory C-type lectin-like receptor NKG2A and killer immunoglobulin-like receptors (KIRs) to interact with MHCs. This is important as loss of MHC type I expression on tumour cells can activate NK cell-mediated lysis and cytokine release, known as “missing-self recognition”. NK cells interact with tumours through receptors (NKG2D, NKp30, NKp44, NKp46), release cytotoxic granules containing perforin and granzymes, induce apoptosis and release pro-inflammatory cytokines (136,137). They have the potential of mass-producing universal donor “off-the-shelf” type treatments, particularly through iPSC-derived NK cells, since patients with solid tumours tolerate allogenic NK cells and do not exhibit GvHD (138). Thus, cancers with low HLA levels are more susceptible to NK therapy, however, those with high HLA expressions tend to be more resistant to treatment. A study on acute myeloid leukaemia suggested that patient and donor KIR-HLA mismatch (alloreactive NK cells) is associated with a reduced relapse rate and increased anti-tumour activity (139). However, in solid tumours, both autologous and allogeneic NK cells have demonstrated efficacy, although only in an OC murine model (140), thus the preference for one or the other depends on individual merits. The limitations of allogeneic therapy are a need for immuno-suppressants and may be limited by subsequent treatments due to antibody generation.
NK cells therapy has been studied to a limited extent in OC. Most studies are in Phase I or II stages and have largely focussed on allogeneic NK treatments, following advances in haematological cancers. For the most part, the therapies are well-tolerated, although with variable NK expansion in vivo (138). The main goal of NK cells therapy is to expand NK cells with molecules/cytokines such as 4-1BBL, IL-2, IL-12, IL-15, IL-18 and IL-21, and maintain the expansion in vivo; this is particularly important if it is to become an “off-the-shelf” treatment. Furthermore, experimenting with NK cells in combination with PD-L1 ICIs, as well as with CAR to enhance anti-tumour efficacy are the latest research strategies (134,141,142). CAR-NK cells have been directed against ovarian CSCs by targeting CD24+, CD44+, and CD133+ cells (143-145). These all exhibited specific cytotoxic activity, especially against CD44+ and CD133+ cells when combined with cisplatin. CAR-NK cells anti-CD24+ have also been effective in primary OC tumours (144), but all treatments still need to uphold within the tumour microenvironment and in clinical trials. The largest study with allogenic NK cells (14 OC participants) had 10 severe adverse events, of which one had tumour lysis syndrome (grade 5) (146). This syndrome is uncommon in solid tumours but is associated with electrolyte abnormalities resulting from high tumour toxicity where their contents are released into the bloodstream, thus patients were subsequently given allopurinol as a prophylaxis. Optimizing NK expansion function in vivo requires further investigations.
Immunomodulators
Immunomodulators are substances, usually drugs, that modify the immune system function by directly targeting key pathways exploited by cancer cells. The most studied immunomodulators in platinum-resistant patients target PD-1/PD-L1 (durvalumab, avelumab, nivolumab, pembrolizumab, atezolizumab) and CTLA-4 (ipilimumab) checkpoints. Most of these studies are phase I or II clinical trials. JAVELIN ovarian 200 was the first phase III trial testing avelumab with or without liposomal doxorubicin, however, found no improvements in PFS or OS (147). Potentially this may reflect the existence of numerous immune evasion mechanisms or that the mutation load in OC is not as high as in melanoma, thus one ICI may not be enough. ICIs have also been associated with adverse events such as myositis, pancreatitis and hypo/hyperthyroidism (148).
Currently, pembrolizumab is FDA-approved for microsatellite-instability high tumours, but not OC. A recent KEYNOTE-100 phase II trial examining pembrolizumab in recurrent OC identified modest results in patients with advanced stage epithelial ovarian OC: objective response rate of 8% and higher responses correlated with higher PD-L1 levels (149). In fact, most PD-1/PD-L1 inhibitors have low response rates (149), thus monotherapy is unlikely to have a significant impact on OC. This is contrasting to other studies where ICIs have been associated with the highest durable responses, a continuous objective response (partial or complete) commencing within 12 months of treatment and lasting ≥6 months, among immunotherapies in melanomas (150,151).
Interestingly, a treatment for OC in BRCA-deficient cells, olaparib, has been associated with robust immune responses in murine models. olaparib treatment was associated with significantly increased intra-tumoural CD4+ and CD8+ T cells, dendritic cell antigen presentation, and reduced MDSCs in spleen, blood and tumoural tissue (152). Furthermore, CD8+ T cells exhibited decreased expressions of co-inhibitory receptors such as PD-1, LAG-3 and TIM-3 in the spleen (152). The anti-tumoural efficacy of PARP inhibition depended on STING pathway activation (152). However, simultaneously, olaparib was associated with increased tumoural expressions of PD-L1 and anti-tumoural effects were only maintained with the addition of a PD-1 antibody (152). The authors suggested this to be a possible mechanism in patients that initially respond well, but later relapse on olaparib chemotherapy, thus suggesting the use of ICIs in addition to PARPi. Although these results should be confirmed in non-animal models, they suggest iatrogenic and “double-edged sword” implications for immunotherapies.
Immunogenic cell death (ICD) inducers
ICD involves the appearance of damage-associated molecular patterns (DAMPs) within the TIME, as a response to cellular trauma or death. DAMPs can be induced through ROS production and ER stress to lead to ICD (153). This stimulates dendritic cells and other antigen presenting cells to produce proinflammatory cytokines and stimulate cytotoxic T-lymphocytes for long-lasting immunity. Developing therapies to induce ICD is one of the more recent priorities in immunotherapy and include both biological (i.e., oncolytic virus) and chemical (i.e., chemotherapeutic drugs, light, ionizing radiation) methods (153).
Oncolytic viruses are engineered to infect tumour cells to cause cell lysis and activate the immune system through secreted pro-inflammatory cytokines, while simultaneously sparing healthy cells and altering the tumour microenvironment. The lysis of a tumorigenic cell can be thought of as an “anti-tumour vaccine” because not only releases progeny virions, but TAAs, NeoAgs, pathogen-associated molecular patterns (PAMPs) and DAMPs. The advantage of this approach is that not all tumour cells must be infected, only a couple to initiate the process. Viruses that have been studied in OC include adeno, vaccinia, Maraba, measles, herpes simplex and reoviruses, which can be combined with other components, such as IL-12 and IL-15 to increase T-lymphocyte responses (154-160). The loss of STING signalling, common in OC, has been associated with increased susceptibility to oncolytic viruses (24), suggesting OC may be susceptible to infections. However, to date, no studies have reached phase III or higher clinical trials, despite some pre-clinical and early phase promising results. More recently, oncolytic viruses have been combined with ICIs. In mouse models, the vaccinia virus induced PD-L1 expression on tumour cells (161). Furthermore, with the addition of a PD-L1 antibody there were increased levels CD4+ and CD8+ T-lymphocytes, IFN-γ, granzyme B and perforin, as well as decreased Treg, MDSC, TAM, exhausted PD-1+CD8+ T-Lymphocytes and viral-induced PD-L1+ dendritic cells (161). Accordingly, tumour burden and survival were improved. Furthermore, oncolytic viruses have been supplemented with transgenes (such as the fusion protein SIRPα-FC) and led to promising results (162). Oncolytic viruses are currently being modified to express bispecific antibodies from infected cancer cells, such as those targeting EpCAM, which can combine oncolysis and T-cell mediated toxicity, while controlling BiTE transcription through viral major late promoter (163). Thus, combination treatments and expressing pro-inflammatory molecules/proteins from infected cancer cells is promising.
Conclusions
Immunotherapies consisting of antibodies, vaccines, ACT, immunomodulators and ICD for OC are still in infancy stages. The harsh TIME remains a barrier and immunotherapies exhibit variable successes between patients. This may be due to hot/cold, genetic, or cellular tumour heterogeneity within a patient, making immunotherapy responses difficult to predict and may require combined therapies. Due to a lack of an OC-specific cell target, most immunotherapies target the same antigens but applying various strategies. Not targeting cellular heterogeneity or mechanisms involved in immune evasion, will not assist in targeting relapse.
Despite these challenges, OC predictably metastasises to the omentum, thus it can be easily targeted with immunotherapies. Strategies recruiting more than one cell type may be more beneficial as the immune system is composed of interactions of multitudes of cell types. To develop better treatments, research is developing macrophage reprogramming and pattern recognition receptor (PRR) agonists. Macrophages are highly plastic cells, and identifying ways to polarize TAMs into pro-inflammatory M1-like cells is beneficial (164). Meanwhile, PRR agonists have gained attention as potential adjuvants, which are substances that enhance immune responses to antigens. PRRs are a group of proteins, mainly receptors on innate and adaptive immune cells, which recognise PAMPs and DAMPs (165). Nevertheless, awareness of immune evasion is critical for future research design. The ideal immunotherapy should be one that withstands the microenvironment, exhibits prolonged responses, has minimal side effects, and is not limited by immune evasion strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hooman Soleymani Majd) for the series “Evolutions in the Management of Advanced Ovarian Cancer” published in Gynecology and Pelvic Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-18/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gpm.amegroups.com/article/view/10.21037/gpm-22-18/coif). The series “Evolutions in the Management of Advanced Ovarian Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. [Crossref] [PubMed]
- Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107-11. [Crossref] [PubMed]
- Cavallo F, De Giovanni C, Nanni P, et al. The immune hallmarks of cancer. In: Cancer Immunology, Immunotherapy. Springer, 2011:319-26.
- Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133-50. [Crossref] [PubMed]
- Guo ZS. The 2018 Nobel Prize in medicine goes to cancer immunotherapy (editorial for BMC cancer). BMC Cancer 2018;18:1086. [Crossref] [PubMed]
- Duan Q, Zhang H, Zheng J, et al. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends in Cancer 2020;6:605-18. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14:9-32. [Crossref] [PubMed]
- Pignata S, Cecere SC, Du Bois A, et al. Treatment of recurrent ovarian cancer. Ann Oncol 2017;28:viii51-6. [Crossref] [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N Engl J Med 2003;348:203-13. [Crossref] [PubMed]
- Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538-43. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ovarian Tumor Tissue Analysis (OTTA) Consortium. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol 2017;3:e173290. [Crossref] [PubMed]
- Mebius RE. Lymphoid organs for peritoneal cavity immune response: milky spots. Immunity 2009;30:670-2. [Crossref] [PubMed]
- Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17:1498-503. [Crossref] [PubMed]
- Phan TG, Croucher PI. The dormant cancer cell life cycle. Nat Rev Cancer 2020;20:398-411. [Crossref] [PubMed]
- Lanitis E, Dangaj D, Irving M, et al. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann Oncol 2017;28:xii18-32. [Crossref] [PubMed]
- Le YS, Kim TE, Kim BK, et al. Alterations of HLA class I and class II antigen expressions in borderline, invasive and metastatic ovarian cancers. Exp Mol Med 2002;34:18-26. [Crossref] [PubMed]
- Shehata M, Mukherjee A, Deen S, et al. Human leukocyte antigen class i expression is an independent prognostic factor in advanced ovarian cancer resistant to first-line platinum chemotherapy. Br J Cancer 2009;101:1321-8. [Crossref] [PubMed]
- Brightwell RM, Grzankowski KS, Lele S, et al. The CD47 "don't eat me signal" is highly expressed in human ovarian cancer. Gynecol Oncol 2016;143:393-7. [Crossref] [PubMed]
- Liu R, Wei H, Gao P, et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget 2017;8:39021-32. [Crossref] [PubMed]
- Li Y, Lu S, Xu Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res 2017;9:2901-10. [PubMed]
- Harrington BS, He Y, Khan T, et al. Anti-CDCP1 immuno-conjugates for detection and inhibition of ovarian cancer. Theranostics 2020;10:2095-114. [Crossref] [PubMed]
- de Queiroz NMGP, Xia T, Konno H, et al. Ovarian cancer cells commonly exhibit defective STING signaling which affects sensitivity to viral oncolysis. Mol Cancer Res 2019;17:974-86. [Crossref] [PubMed]
- Liu D, Wu H, Wang C, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ 2019;26:1735-49. [Crossref] [PubMed]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type i interferon-dependent innate immunity. Nature 2009;461:788-92. [Crossref] [PubMed]
- Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014;41:843-52. [Crossref] [PubMed]
- Lam E, Falck-Pedersen E. Unabated Adenovirus Replication following Activation of the cGAS/STING-Dependent Antiviral Response in Human Cells. J Virol 2014;88:14426-39. [Crossref] [PubMed]
- Zhang J, Chen Y, Chen X, et al. Deubiquitinase USP35 restrains STING-mediated interferon signaling in ovarian cancer. Cell Death Differ 2021;28:139-55. [PubMed]
- Ghaffari A, Peterson N, Khalaj K, et al. Sting agonist therapy in combination with pd-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer 2018;119:440-9. [Crossref] [PubMed]
- Huang RY, Eppolito C, Lele S, et al. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015;6:27359-77. [Crossref] [PubMed]
- Imai Y, Hasegawa K, Matsushita H, et al. Expression of multiple immune checkpoint molecules on t cells in malignant ascites from epithelial ovarian carcinoma. Oncol Lett 2018;15:6457-68. [Crossref] [PubMed]
- Mulati K, Hamanishi J, Matsumura N, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer 2019;120:115-27. [Crossref] [PubMed]
- Qin S, Xu L, Yi M, et al. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol Cancer 2019;18:155. [Crossref] [PubMed]
- Cai D, Li J, Liu D, et al. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol 2020;17:227-36. [Crossref] [PubMed]
- Chen F, Xu Y, Chen Y, et al. TIGIT enhances CD4+ regulatory T-cell response and mediates immune suppression in a murine ovarian cancer model. Cancer Med 2020;9:3584-91. [Crossref] [PubMed]
- Tu L, Guan R, Yang H, et al. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int J Cancer 2020;147:423-39. [Crossref] [PubMed]
- Parvathareddy SK, Siraj AK, Al-Badawi IA, et al. Differential expression of PD-L1 between primary and metastatic epithelial ovarian cancer and its clinico-pathological correlation. Sci Rep 2021;11:3750. [Crossref] [PubMed]
- Li X, Liu Y, Zheng S, et al. Role of exosomes in the immune microenvironment of ovarian cancer. Oncol Lett 2021;21:37. [Crossref] [PubMed]
- Shang B, Liu Y, Jiang SJ, et al. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep 2015;5:15179. [Crossref] [PubMed]
- Drakes ML, Stiff PJ. Regulation of ovarian cancer prognosis by immune cells in the tumor microenvironment. Cancers (Basel) 2018;10:302. [Crossref] [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [Crossref] [PubMed]
- Zhang S, Ke X, Zeng S, et al. Analysis of CD8+ Treg cells in patients with ovarian cancer: A possible mechanism for immune impairment. Cell Mol Immunol 2015;12:580-91. [Crossref] [PubMed]
- Yang M, Zhang G, Wang Y, et al. Tumour-associated neutrophils orchestrate intratumoural IL-8-driven immune evasion through Jagged2 activation in ovarian cancer. Br J Cancer 2020;123:1404-16. [Crossref] [PubMed]
- Wang Y, Xu RC, Zhang XL, et al. Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine 2012;59:145-55. [Crossref] [PubMed]
- Sperner-Unterweger B, Neurauter G, Klieber M, et al. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiology 2011;216:296-301. [Crossref] [PubMed]
- Qian F, Villella J, Wallace PK, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res 2009;69:5498-504. [Crossref] [PubMed]
- Sinclair LV, Neyens D, Ramsay G, et al. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat Commun 2018;9:1981. [Crossref] [PubMed]
- Mezrich JD, Fechner JH, Zhang X, et al. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J Immunol 2010;185:3190-8. [Crossref] [PubMed]
- Duluc D, Delneste Y, Tan F, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007;110:4319-30. [Crossref] [PubMed]
- Kawamura K, Komohara Y, Takaishi K, et al. Detection of M2 macrophages and colony-stimulating factor 1expression in serous and mucinous ovarian epithelial tumors. Pathol Int 2009;59:300-5. [Crossref] [PubMed]
- Bai Y, Yin K, Su T, et al. CTHRC1 in Ovarian Cancer Promotes M2-Like Polarization of Tumor-Associated Macrophages via Regulation of the STAT6 Signaling Pathway. Onco Targets Ther 2020;13:5743-53. [Crossref] [PubMed]
- Zhang Q, Cai DJ, Li B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol Med Rep 2015;11:4685-93. [Crossref] [PubMed]
- Macciò A, Gramignano G, Cherchi MC, et al. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep 2020;10:6096. [Crossref] [PubMed]
- Hu Z, Cunnea P, Zhong Z, et al. The oxford classic links epithelial-to-mesenchymal transition to immunosuppression in poor prognosis ovarian cancers. Clin Cancer Res 2021;27:1570-9. [Crossref] [PubMed]
- Yin M, Li X, Tan S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J Clin Invest 2016;126:4157-73. [Crossref] [PubMed]
- Wertel I, Surówka J, Polak G, et al. Macrophage-derived chemokine CCL22 and regulatory T cells in ovarian cancer patients. Tumour Biol 2015;36:4811-7. [Crossref] [PubMed]
- Schutyser E, Struyf S, Proost P, et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem 2002;277:24584-93. [Crossref] [PubMed]
- Gottlieb CE, Mills AM, Cross JV, et al. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: A comparison of matched primary and metastatic tumors. Gynecol Oncol 2017;144:607-12. [Crossref] [PubMed]
- Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 2006;203:871-81. [Crossref] [PubMed]
- Bapat SA, Mali AM, Koppikar CB, et al. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 2005;65:3025-9. [Crossref] [PubMed]
- Zhang S, Balch C, Chan MW, et al. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res 2008;68:4311. [Crossref] [PubMed]
- Lupia M, Cavallaro U. Ovarian cancer stem cells: Still an elusive entity? Mol Cancer 2017;16:64. [Crossref] [PubMed]
- Deng X, Zhang P, Liang T, et al. Ovarian cancer stem cells induce the M2 polarization of macrophages through the PPARγ and NF-κB pathways. Int J Mol Med 2015;36:449-54. [Crossref] [PubMed]
- Alvero AB, Fu HH, Holmberg J, et al. CANCER STEM CELLS Stem-Like Ovarian Cancer Cells Can Serve as Tumor Vascular Progenitors. Stem Cells 2009;27:2405-13. [Crossref] [PubMed]
- Hu L, Mcarthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer 2010;102:1276-83. [Crossref] [PubMed]
- Silva IA, Bai S, McLean K, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res 2011;71:3991-4001. [Crossref] [PubMed]
- Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017;168:692-706. [Crossref] [PubMed]
- Song M, Sandoval TA, Chae CS, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 2018;562:423-8. [Crossref] [PubMed]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015;161:1527-38. [Crossref] [PubMed]
- Veglia F, Tyurin VA, Blasi M, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019;569:73-8. [Crossref] [PubMed]
- Cui T, Srivastava AK, Han C, et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1. Cell Death Dis 2018;9:561. [Crossref] [PubMed]
- Hu Z, Artibani M, Alsaadi A, et al. The Repertoire of Serous Ovarian Cancer Non-genetic Heterogeneity Revealed by Single-Cell Sequencing of Normal Fallopian Tube Epithelial Cells. Cancer Cell 2020;37:226-242.e7. [Crossref] [PubMed]
- Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am 1978;58:131-42. [Crossref] [PubMed]
- Alberts DS, Green S, Hannigan EV, et al. Improved therapeutic index of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide: final report by the Southwest Oncology Group of a phase III randomized trial in stages III and IV ovarian cancer. J Clin Oncol 1992;10:706-17. [Crossref] [PubMed]
- Kampan NC, Madondo MT, McNally OM, et al. Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int. 2015;2015:413076. [Crossref] [PubMed]
- AstraZeneca. FDA approved olaparib (LYNPARZA, AstraZeneca Pharmaceuticals LP) for the maintenance treatment of adult patients with deleterious or suspected deleterious germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube or p [Internet]. Press Release. 2018 [cited 2021 Jun 11]. Available online: https://www.fda.gov/drugs/fda-approved-olaparib-lynparza-astrazeneca-pharmaceuticals-lp-maintenance-treatment-adult-patients
- GSK. FDA approves Zejula (niraparib) as the only once-daily PARP inhibitor in first-line monotherapy maintenance treatment for women with platinum-responsive advanced ovarian cancer regardless of biomarker status [Internet]. Press Release. 2020 [cited 2021 Jun 11]. Available online: https://www.gsk.com/en-gb/media/press-releases/fda-approves-parp-inhibitor-in-first-line-monotherapy-maintenance-treatment-for-women-with-platinum-responsive-advanced-ovarian-cancer-regardless-of-biomarker-status/
- Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol 2018;19:1680-7. [Crossref] [PubMed]
- Lo CS, Sanii S, Kroeger DR, et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res 2017;23:925-34. [Crossref] [PubMed]
- Lee EK, Liu JF. Antibody-drug conjugates in gynecologic malignancies. Gynecol Oncol 2019;153:694-702. [Crossref] [PubMed]
- Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required ? Br J Cancer 2017;117:1736-42. [Crossref] [PubMed]
- Khongorzul P, Ling CJ, Khan FU, et al. Antibody-Drug Conjugates: A Comprehensive Review. Mol Cancer Res 2020;18:3-19. [Crossref] [PubMed]
- Moore KN, Vergote I, Oaknin A, et al. FORWARD I: a Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol 2018;14:1669-78. [Crossref] [PubMed]
- Moore K, Oza A, Colombo N, et al. FORWARD I (GOG 3011): A phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients (pts) with platinum-resistant ovarian cancer (PROC). Ann Oncol 2019;30:v403. [Crossref]
- Ab O, Whiteman KR, Bartle LM, et al. IMGN853, a Folate Receptor-α (FRα)-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRα-Expressing Tumors. Mol Cancer Ther 2015;14:1605-13. [Crossref] [PubMed]
- Rios-Doria J, Harper J, Rothstein R, et al. Antibody-Drug Conjugates Bearing Pyrrolobenzodiazepine or Tubulysin Payloads Are Immunomodulatory and Synergize with Multiple Immunotherapies. Cancer Res 2017;77:2686-98. [Crossref] [PubMed]
- Zhong H, Chen C, Tammali R, et al. Improved Therapeutic Window in BRCA-mutant Tumors with Antibody-linked Pyrrolobenzodiazepine Dimers with and without PARP Inhibition. Mol Cancer Ther 2019;18:89-99. [Crossref] [PubMed]
- Loganzo F, Sung M, Gerber HP. Mechanisms of Resistance to Antibody-Drug Conjugates. Mol Cancer Ther 2016;15:2825-34. [Crossref] [PubMed]
- Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer 2010;127:2209-21. [Crossref] [PubMed]
- English DP, Bellone S, Schwab CL, et al. Solitomab, an epithelial cell adhesion molecule/CD3 bispecific antibody (BiTE), is highly active against primary chemotherapy-resistant ovarian cancer cell lines in vitro and fresh tumor cells ex vivo. Cancer 2015;121:403-12. [Crossref] [PubMed]
- Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell - Engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29:2493-8. [Crossref] [PubMed]
- Slaney CY, Wang P, Darcy PK, et al. CARs versus biTEs: A comparison between T cell–redirection strategies for cancer treatment. Cancer Discovery 2018;8:924-34. [Crossref] [PubMed]
- Rousseau RF, Hirschmann-Jax C, Takahashi S, et al. Cancer vaccines. Hematol Oncol Clin North Am 2001;15:741-73. [Crossref] [PubMed]
- Tanyi JL, George E. Personalized vaccination against ovarian cancer: what are the possibilities? Expert Review of Vaccines 2018;17:955-8. [Crossref] [PubMed]
- Garcia-Soto AE, Schreiber T, Strbo N, et al. Cancer-testis antigen expression is shared between epithelial ovarian cancer tumors. Gynecol Oncol 2017;145:413-9. [Crossref] [PubMed]
- Szender JB, Papanicolau-Sengos A, Eng KH, et al. NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer. Gynecol Oncol 2017;145:420. [Crossref] [PubMed]
- Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003;63:6076-83. [PubMed]
- Woloszynska-Read A, Mhawech-Fauceglia P, Yu J, et al. Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clin Cancer Res 2008;14:3283. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Martin SD, Brown SD, Wick DA, et al. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS One 2016;11:e0155189. [Crossref] [PubMed]
- Bobisse S, Genolet R, Roberti A, et al. Sensitive and frequent identification of high avidity neo-epitope specific CD8 + T cells in immunotherapy-naive ovarian cancer. Nat Commun 2018;9:1092. [Crossref] [PubMed]
- Liu S, Matsuzaki J, Wei L, et al. Efficient identification of neoantigen-specific T-cell responses in advanced human ovarian cancer. J Immunother Cancer 2019;7:156. [Crossref] [PubMed]
- O’Donnell T, Christie EL, Ahuja A, et al. Chemotherapy weakly contributes to predicted neoantigen expression in ovarian cancer. BMC Cancer 2018;18:87. [Crossref] [PubMed]
- Sarivalasis A, Boudousquié C, Balint K, et al. A Phase I/II trial comparing autologous dendritic cell vaccine pulsed either with personalized peptides (PEP-DC) or with tumor lysate (OC-DC) in patients with advanced high-grade ovarian serous carcinoma. J Transl Med 2019;17:391. [Crossref] [PubMed]
- Chiang CLL, Kandalaft LE, Tanyi J, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin Cancer Res 2013;19:4801-15. [Crossref] [PubMed]
- Prokopowicz ZM, Arce F, Biedron R, et al. Hypochlorous Acid: A Natural Adjuvant That Facilitates Antigen Processing, Cross-Priming, and the Induction of Adaptive Immunity. J Immunol 2010;184:824-35. [Crossref] [PubMed]
- Tanyi JL, Bobisse S, Ophir E, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med 2018;10:5931. [Crossref] [PubMed]
- Cibula D, Rob L, Mallmann P, et al. Dendritic cell-based immunotherapy (DCVAC/OvCa) with chemotherapy in patients with platinum-sensitive, relapsed, epithelial ovarian carcinoma: Survival analysis of a phase II, open-label, randomized, multicenter trial (study SOV02). Gynecol Oncol 2019;154:18. [Crossref]
- Zacharakis N, Chinnasamy H, Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018;24:724-30. [Crossref] [PubMed]
- Goode EL, Block MS, Kalli KR, et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol 2017;3:e173290. [Crossref] [PubMed]
- van den Berg JH, Heemskerk B, van Rooij N, et al. Tumor infiltrating lymphocytes (TIL) therapy in metastatic melanoma: boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer 2020;8:e000848. [Crossref] [PubMed]
- Fujita K, Ikarashi H, Takakuwa K, et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res 1995;1:501-7. [PubMed]
- Aoki Y, Takakuwa K, Kodama S, et al. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res 1991;51:1934-9. [PubMed]
- Freedman RS, Edwards CL, Kavanagh JJ, et al. Intraperitoneal adoptive immunotherapy of ovarian carcinoma with tumor-infiltrating lymphocytes and low-dose recombinant interleukin-2: a pilot trial. J Immunother Emphasis Tumor Immunol 1994;16:198-210. [Crossref] [PubMed]
- Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015;16:807-20. [Crossref] [PubMed]
- Scheper W, Kelderman S, Fanchi LF, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 2019;25:89-94. [Crossref] [PubMed]
- Friese C, Harbst K, Borch TH, et al. CTLA-4 blockade boosts the expansion of tumor-reactive CD8+ tumor-infiltrating lymphocytes in ovarian cancer. Sci Rep 2020;10:3914. [Crossref] [PubMed]
- Matsuzaki J, Tsuji T, Chodon T, et al. A rare population of tumor antigen-specific CD4+CD8+ double-positive αβ T lymphocytes uniquely provide CD8-independent TCR genes for engineering therapeutic T cells. J Immunother Cancer 2019;7:7. [Crossref] [PubMed]
- Deniger DC, Pasetto A, Robbins PF, et al. T-cell Responses to TP53 "Hotspot" Mutations and Unique Neoantigens Expressed by Human Ovarian Cancers. Clin Cancer Res 2018;24:5562-73. [Crossref] [PubMed]
- Matsuda T, Leisegang M, Park JH, et al. Induction of neoantigen-specific cytotoxic T cells and construction of T-cell receptor-engineered T cells for ovarian cancer. Clin Cancer Res 2018;24:5357-67. [Crossref] [PubMed]
- Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 2017;7:10541. [Crossref] [PubMed]
- Benard E, Casey NP, Inderberg EM, et al. SJI 2020 special issue: A catalogue of Ovarian Cancer targets for CAR therapy. Scand J Immunol 2020;92:e12917. [Crossref] [PubMed]
- Ang WX, Li Z, Chi Z, et al. Intraperitoneal immunotherapy with T cells stably and transiently expressing anti-EpCAM CAR in xenograft models of peritoneal carcinomatosis. Oncotarget 2017;8:13545-59. [Crossref] [PubMed]
- Fu J, Shang Y, Qian Z, et al. Chimeric antigen receptor-t (Car-t) cells targeting epithelial cell adhesion molecule (epcam) can inhibit tumor growth in ovarian cancer mouse model. J Vet Med Sci 2021;83:241-7. [Crossref] [PubMed]
- Tanyi JL, Stashwick C, Plesa G, et al. Possible compartmental cytokine release syndrome in a patient with recurrent ovarian cancer after treatment with mesothelin-targeted CAR-T cells. J Immunother 2017;40:104-7. [Crossref] [PubMed]
- Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013;31:71-5. [Crossref] [PubMed]
- Lanitis E, Poussin M, Klattenhoff AW, et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res 2013;1:43-53. [Crossref] [PubMed]
- Juillerat A, Marechal A, Filhol JM, et al. An oxygen sensitive self-decision making engineered CAR T-cell. Sci Rep 2017;7:39833. [Crossref] [PubMed]
- Kim KH, Dmitriev I, O’Malley JP, et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res 2012;18:3440-51. [Crossref] [PubMed]
- Hoogstad-van Evert JS, Maas RJ, Van Der Meer J, et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018;9:34810-20. [Crossref] [PubMed]
- Webb JR, Milne K, Watson P, et al. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker cd103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 2014;20:434-44. [Crossref] [PubMed]
- Li Y, Hermanson DL, Moriarity BS, et al. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018;23:181-192.e5. [Crossref] [PubMed]
- Hermanson DL, Bendzick L, Pribyl L, et al. Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem Cells 2016;34:93-101. [Crossref] [PubMed]
- Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J 2004;23:255. [Crossref] [PubMed]
- Nersesian S, Glazebrook H, Toulany J, et al. Naturally Killing the Silent Killer: NK Cell-Based Immunotherapy for Ovarian Cancer. Front Immunol 2019;10:1782. [Crossref] [PubMed]
- Hoogstad-van Evert JS, Bekkers R, Ottevanger N, et al. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecologic Oncology 2020;157:810-6. [Crossref] [PubMed]
- Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: Challenging its predictive value. Blood 2007;110:433-40. [Crossref] [PubMed]
- Poznanski SM, Nham T, Chew MV, et al. Expanded CD56superbrightCD16+ NK cells from ovarian cancer patients are cytotoxic against autologous tumor in a patient-derived xenograft murine model. Cancer Immunol Res 2018;6:1174-85. [Crossref] [PubMed]
- Cao B, Liu M, Wang L, et al. Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer. Biochem Biophys Res Commun 2020;524:96-102. [Crossref] [PubMed]
- Oyer JL, Gitto SB, Altomare DA, et al. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 2018;7:e1509819. [Crossref] [PubMed]
- Klapdor R, Wang S, Hacker U, et al. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum Gene Ther 2017;28:886-96. [Crossref] [PubMed]
- Klapdor R, Wang S, Morgan M, et al. Characterization of a novel third-generation anti-CD24-CAR against ovarian cancer. Int J Mol Sci 2019;20:660. [Crossref] [PubMed]
- Klapdor R, Wang S, Morgan MA, et al. NK Cell-Mediated Eradication of Ovarian Cancer Cells with a Novel Chimeric Antigen Receptor Directed against CD44. Biomedicines 2021;9:1339. [Crossref] [PubMed]
- Geller MA, Cooley S, Judson PL, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011;13:98-107. [Crossref] [PubMed]
- Pujade-Lauraine E, Fujiwara K, Dychter SS, et al. Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 Phase III study design. Future Oncol 2018;14:2103-13. [Crossref] [PubMed]
- Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol Oncol 2019;152:243-50. [Crossref] [PubMed]
- Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 2019;30:1080-7. [Crossref] [PubMed]
- Kaufman HL, Andtbacka RHI, Collichio FA, et al. Durable response rate as an endpoint in cancer immunotherapy: Insights from oncolytic virus clinical trials. J Immunother Cancer 2017;5:72. [Crossref] [PubMed]
- Pons-Tostivint E, Latouche A, Vaflard P, et al. Comparative Analysis of Durable Responses on Immune Checkpoint Inhibitors Versus Other Systemic Therapies: A Pooled Analysis of Phase III Trials. JCO Precis Oncol 2019;3:1-10. [Crossref] [PubMed]
- Ding L, Kim HJ, Wang Q, et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 2018;25:2972-2980.e5. [Crossref] [PubMed]
- Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012;12:860-75. [Crossref] [PubMed]
- Santos JM, Heiniö C, Cervera-Carrascon V, et al. Oncolytic adenovirus shapes the ovarian tumor microenvironment for potent tumor-infiltrating lymphocyte tumor reactivity. J Immunother Cancer 2020;8:e000188. [Crossref] [PubMed]
- Horita K, Kurosaki H, Nakatake M, et al. lncRNA UCA1-Mediated Cdc42 Signaling Promotes Oncolytic Vaccinia Virus Cell-to-Cell Spread in Ovarian Cancer. Mol Ther-Oncolytics 2019;13:35-48. [Crossref] [PubMed]
- McGray AJR, Huang RY, Battaglia S, et al. Oncolytic Maraba virus armed with tumor antigen boosts vaccine priming and reveals diverse therapeutic response patterns when combined with checkpoint blockade in ovarian cancer. J Immunother Cancer 2019;7:189. [Crossref] [PubMed]
- Galanis E, Atherton PJ, Maurer MJ, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res 2015;75:22-30. [Crossref] [PubMed]
- Cohn DE, Sill MW, Walker JL, et al. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2017;146:477-83. [Crossref] [PubMed]
- Thomas ED, Meza-Perez S, Bevis KS, et al. IL-12 Expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice. J Ovarian Res 2016;9:70. [Crossref] [PubMed]
- Kowalsky SJ, Liu Z, Feist M, et al. Superagonist IL-15-Armed Oncolytic Virus Elicits Potent Antitumor Immunity and Therapy That Are Enhanced with PD-1 Blockade. Mol Ther 2018;26:2476-86. [Crossref] [PubMed]
- Liu Z, Ravindranathan R, Kalinski P, et al. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun 2017;8:14754. [Crossref] [PubMed]
- Huang Y, Lv S, Liu P, et al. A SIRPα-Fc fusion protein enhances the antitumor effect of oncolytic adenovirus against ovarian cancer. Mol Oncol 2020;14:657-68. [Crossref] [PubMed]
- Freedman JD, Hagel J, Scott EM, et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol Med 2017;9:1067-87. [Crossref] [PubMed]
- Hu G, Su Y, Kang BH, et al. High-throughput phenotypic screen and transcriptional analysis identify new compounds and targets for macrophage reprogramming. Nat Commun 2021;12:773. [Crossref] [PubMed]
- Aleynick M, Svensson-Arvelund J, Flowers CR, et al. Pathogen Molecular Pattern Receptor Agonists: Treating Cancer by Mimicking Infection. Clin Cancer Res 2019;25:6283-94. [Crossref] [PubMed]
Cite this article as: Romanchik D, Albukhari A, Artibani M, Ahmed AA. Role of immunotherapy in ovarian cancer: a narrative review. Gynecol Pelvic Med 2022;5:33.


